-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2020; 10(1): 1-8
doi:10.5923/j.ajee.20201001.01

Evaluation of Biosorptive Capacity of Waste Watermelon Seed for Lead (II) Removal from Aqueous Solution
B. K. Adeoye 1, B. A. Akinbode 1, J. D Awe 1, C. T. Akpa 2
1Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria
2Food Department, Federal Institute of Industrial Research, Oshodi, Lagos, Nigeria
Correspondence to: B. K. Adeoye , Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The efficacy of grinded watermelon seed as alternative low-cost biosorbents for the removal of Pb(II) ions from aqueous solutions was investigated. Batch adsorption studies were carried out by preparing a simulated waste sample and effects of adsorbent dose, temperature, initial metal concentration, contact time and pH on the percentage Pb(II) removal were evaluated. The Freundlich constant (n) and separation factor (RL) values suggest that the lead ions was favourably adsorbed onto biosorbents. Pseudo-second order kinetics best described the process with R2 (0.997). The results of pseudo second order kinetic model for the adsorption of lead showed straight line graph which starts from the origin. The value 1/n greater than 1 is indicative of cooperative adsorption and Freundlich isotherm. Watermelon seed has viable characteristics for preparing biosorbents.
Keywords: Biosorption isotherm, Kinetics, Lead (II), Agricultural waste, Biosorption isotherms, Watermelon seed, Thermodynamic properties
Cite this paper: B. K. Adeoye , B. A. Akinbode , J. D Awe , C. T. Akpa , Evaluation of Biosorptive Capacity of Waste Watermelon Seed for Lead (II) Removal from Aqueous Solution, American Journal of Environmental Engineering, Vol. 10 No. 1, 2020, pp. 1-8. doi: 10.5923/j.ajee.20201001.01.
Article Outline
1. Introduction
- Unavailability of clean water for drinking is a great challenge in most of developing countries of the world due to daily contamination by excessive release of heavy metals into the environment due to industrialization and urbanization [14]. Industrial uses of metals and other domestic processes have introduced substantial amounts of potentially toxic heavy metals into the atmosphere and into the aquatic and terrestrial environments. Heavy metals are elements having atomic weights between 63.5 and 200.6, and a specific gravity greater than 5.0 [11]. They generally refers to the elements such as Cd (cadmium), Cr (chromium), Cu (copper), Hg (mercury), Ni (nickel), Pb (lead), Fe (ferum) and Zn (zinc) which are commonly associated with pollution and toxicity problems. Unlike organic pollutants, the majority of which are susceptible to biological degradation, heavy metal ions do not degrade into harmless end products [17]. They occur naturally in rock formation and ore minerals and so a range of normal background concentration is associated with each of these elements in soils, sediments, waters and living organisms [10;24]. In small quantities, certain heavy metals are nutritionally essential for healthy life. Some of these are referred to as the trace elements (e.g., iron, copper, manganese, and zinc). These elements, or some form of them, are commonly found naturally in foodstuffs, in fruits and vegetables, and in commercially available multivitamin products [16]. Heavy metals are also common in industrial applications such as in the manufacture of pesticides, batteries, alloys, electroplated metal parts, textile dyes, steel, mining, refining ores, fertilizers industries, paper industries and so forth [12]. Many of these products are in our homes and actually add to our quality of life when properly used.Lead, being among the most dangerous heavy metal is a group IV element on the periodic table which is remarkably highly resistant to corrosion in most acid and naturally occur as element buried in the earth crust in insoluble and biologically inoffensive forms [9]. Exposure to lead is widely recognized as a major risk factor for several human diseases once it goes beyond the World Health Organisation (WHO) maximum permissible limit (3 - 10μg·L-1) in drinking water [20;22]. It forms complexes with oxo-groups in enzymes to affect virtually all steps in the process of haemoglobin synthesis and porphyrin metabolism [1]. Other problems associated with toxic levels of lead exposure are encephalopathy, seizures and mental retardation, anemia and nephropathy [18]. Hence, lead must be removed as much as possible from industrial effluents to prevent environmental hazard from its discharge.Various techniques have been applied for the removal of lead from water. Quite a number of studies have utilized filtration, ion exchange and chemical precipitation [11]. These techniques have been reported to be economically disadvantage due to high cost of their execution. Adsorption technology has been proposed as a cost-effective water decontamination techniques over traditional methods such as coagulation, flocculation, filtration, ozonation or sedimentation [23]. A number of adsorption materials have been studied for their capacity to remove pollutants, including ion exchange resins, bentonite, zeolite [8], silica gel, activated carbon and alginate [5]. Activated carbon is the most widely used adsorbent with high adsorption capacity for organic compounds, a result of their high surface area and surface reactivity but their regeneration is, however, difficult and expensive [6;25].In search of an effective and economical technology have brought about biosorption. A technology that is based on metal binding capacities of various biological materials such as agricultural waste which contains a variety of organic compounds of cellulose, hemicelluloses and lignin that make them effective adsorbents for a wide range of pollutants due to the presence of functional groups such as hydroxyl, carboxyl, methoxyl and phenols [12a;12b]. Agricultural waste normally contains a variety of organic compounds (lignin, cellulose and hemicelluloses) and functional groups (hydroxyl, carbonyl and amino). Both organic compounds and functional groups have great affinity for metal ion complexation [24]. Low cost agricultural wastes by-product such as tea waste, coffee waste, kapok fibre, lam tree (Cordia africana) sawdust, Ricinus communis and coir fibre have been tested for metal ion adsorption by different reseachers [3;4;26]. [37] carried out optimization and intensification methodology for the simultaneous isolation of biophenols from agricultural waste, using imprinted materials and nanofiltration membranes. The predictive mathematical models for the adsorption dynamics and the membrane separation resulted in a significant reduction of the carbon footprint, E factor, and economic sustainability. Also, [38] work on nonwoven composite membrane supports made from three renewable materials: bamboo fiber, poly(lactic acid) (PLA), and dimethyl carbonate. The biobased membrane supports exhibited a porous structure (reduced porosity) with high tensile strengths comparable to conventional materials, such as polypropylene. Effective use of biomass wastes has become one of the promising fields of the treatment of heavy metals due to availability in large quantities, renewable in nature, eco-friendly [21]. In seeds categories, different agricultural waste seeds have been researched as potential alternative to watermelon seed as adsorbent in waste water treatment. [33] investigated the adsorption of copper ions onto Capsicum annuum (red pepper) seeds. The nature of the possible adsorbent and metal ion interactions was examined by the FTIR technique. They concluded that the adsorption of copper(II) ions onto C. annuum seeds could be described by the pseudo-second-order kinetic model and also followed the intraparticle diffusion model up to 60 min, but diffusion was not only the rate controlling step. Also, [36] developed activated carbon from grape seeds and was tested in the adsorption of diuron from aqueous phase. The result of their work showed high absorption efficiency. Furthermore, [39] used Jatropha curcas seeds (JS) as the raw material for the production of activated carbon by simple thermo-chemical activation with NaOH as a chemical activating agent and were tested for the single-component adsorption study using the gravimetric adsorption method. Results from the experiments showed that adsorption by activated carbon increased with increasing number of chlorine atoms and hence, with increasing molecular weight. In essence, an adsorbent can be regarded as a low cost adsorbent if it is readily available and requires little processing, or is a waste material or by-product from another industry. In exploring these opportunity, there is an urgent need that all possible sources of agro-based inexpensive adsorbents should be explored and their potential for the removal of heavy metals should be studied in detail. The objective of this study is to contribute in the search for less expensive watermelon seed as adsorbents, which are in many cases also pollution sources.Watermelon seeds are generated in large amount as waste product after the consumption of watermelon and these constitute environmental menace due to the fact that they are left to decompose being of non-economic importance. However, watermelon seed is a rich lignocellulosic agricultural waste whose previous application has been limited to grinding into coarse flower for oils extraction [7] and not yet applied to Pb(II) removal. The present study investigated the adsorptive efficacy of watermelon seed as a low-cost adsorbent for the biosorption of Lead (II) from aqueous solution. The effects of physical parameters such as initial concentration, pH, and temperature and biosorbent dosage on the biosorption process have been investigated. In addition, the biosorption equilibrium isotherms, kinetics and thermodynamic parameters have been determined.
2. Materials and Methods
2.1. Preparation and Characterization of Adsorbent
- The watermelon seed used were obtained from Oba Market, Akure Ondo State, Nigeria. The Watermelon seed were thoroughly washed under tap water and then distilled water to remove contaminants and allowed to dry for a while in air. The partially moist watermelon seed were then taken to the laboratory and oven dried for 24 hours at 100°C to constant weight before being grounded and screened to 25 - 63 μm mesh particle size. It was then stored stored in a desiccator for further usage in the experiment.
2.2. Preparation of Simulated Wastewater
- Stock solution 1000ppm of pb2+ was prepared by dissolving 1.60g of lead (II) Nitrites pb(NO3)2 in 1L of distilled water and this was used to prepare a simulated waste water sample. All other reagents used were of analytical grade and deionized water was used in solution preparation. Other concentrations (20 - 100 mg/L) were obtained from this stock solution by serial dilution. Fresh dilutions were used for each experiment. The concentration of Pb(II) in simulated wastewater was analysed by Atomic Absorption Spectrophotometer (model PyeUnicam SP-9 Cambridge, UK). The working solution was prepared by a modified method of [28]. A stock solution i.e 1000ppm of pb2+ was prepared using the relation in equation;
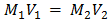 | (1) |
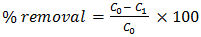 | (2) |
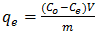 | (3) |
2.3. Biosorption Equilibrium Studies
- Equilibrium tests were carried out on the adsorption of Pb(II) on watermelon seed. The effect of initial metal ion concentration, contact time, temperature, solution pH and adsorbent dose were investigated. Sample solutions were withdrawn at time interval and equilibrium to determine residual concentrations. Solutions were filtered prior to analysis in order to minimise the interference of the watermelon seed. For equilibrium studies, the experiment was carried out for 360 minutes to ensure that equilibrium was reached.
2.4. Effects of Adsorption Parameters
- Powdered adsorbent (grinded watermelon seed), 0.5g was measured into six different 125 ml Erlenmeyer flask with 50ppm of Pb(II) in 1000ml solution with pH of 7 monitored using a pH meter (model CD70 WPA) and controlled with 1M of NaOH and HCL and these six different samples were analysed for pH 1, 2, 4, and 5. The mixture was agitated for 6 hours at 30°C at a constant 125 rpm using the Thermostatic water bath shaker and the supernatant was filtered using Watman Filter Paper and Lead (II) concentration was analysed using Atomic Absorption Spectrophotometer (AAS). The effect of contact time (5, 15, 30, 60, 90, 120, 180, 240, 300 and 360 minutes), biosorbent dose (0.2, 0.5, 0.7 and 1.0 g), initial metal ion concentration (20, 50, 100, 150, 200 ppm) and temperature (28°C, 40°C, 50°C and 60°C) were evaluated during the study. During the experiment, the particular parameter considered was varied while the other four were kept constant variously for watermelon seed biosorbents.
3. Results and Discussion
3.1. Effects of pH
- pH is a very important parameter in adsorption studies as it affect surface charges on the adsorbent and degree of ionization of the sorbate . From the Figure 1, it was observed that lead was adsorbed at pH values of 1, 2, 3, 4, 5 at 75%, 80%, 59.6%, 62% and 55.1% respectively. There was increase in the adsorption of lead on watermelon seed powder but the maximum adsorption was gotten at pH value 2 with 80% before the downward trend of the profile. These variation in the results may be due to the adsorbent or environmental conditions. It can be inferred that the removal of pb2+ from the wastewater using the watermelon seed powder biosorbent is not only by adsorption but also by the precipitation phenomena [8;27].
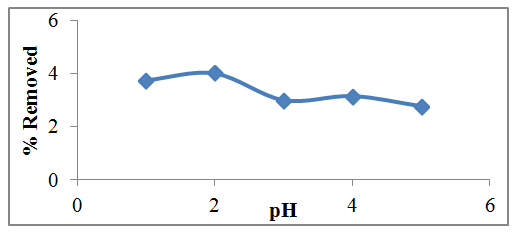 | Figure 1. Effect of pH on the percentage removal of Lead (II) using biosorbents |
3.2. Effect of Initial Concentration and Contact time
- The rate of biosorption is a function of the initial concentration and contact time of the sorbate and these makes them important factors for effective biosorption. Figure 2 shows the effect of contact time on Pb(II) adsorption onto watermelon seed. The quantity of lead adsorbed at 5, 15, 30, 60, 90, 120, 180, 240, 300, 360 mins were 69.1, 71.56, 75.9, 76.08, 80.2, 80.04, 79.48, 78.64, 78.56 and 78.02% respectively. It was observed that the adsorption of Pb(II) by the watermelon seed increased with contact time until equilibrium was reached at around 90 mins. The biosorption process was rapid initially due to increased availability of active binding site on the biosorbent surface and this is controlled by diffusion process from the bulk to the surface. However, during the last stages, biosorption is likely to be attachment controlled process due to reduction in the available active sites. Watermelon seed has 80.2% removal at equilibrium but after 90 mins, at 120 mins the adsorption decreases significantly. Figure 3 reveals that, at lower concentrations of 20 - 50mg/L, biosorption was rapid at about 60 minutes while at higher concentration, the biosorption process became constant at 120 mins as observed in Figures 3. At lower concentrations, all metal ions present in the solution interacted with binding sites and then facilitated about 94.9% on 20 mg/L at initial concentration. At higher concentrations between 80 - 120 mg/L, more Pb(II) is left unabsorbed in the solution due to the saturation of binding sites. This may be due to the increase in the number of ions competing for available binding sites on the watermelon considered in this study. This result is similar to other researcher findings [22].
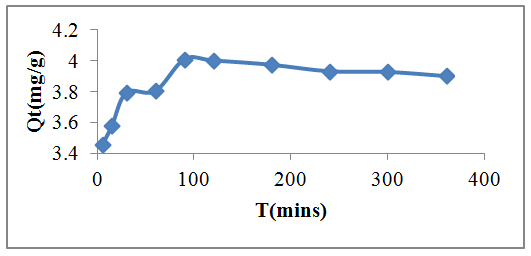 | Figure 2. Effect of contact time on the percentage removal of Lead(II) from a 100ml aqueous solution containing 0.5g of the watermelon seed powder biosorbent at different time intervals for 6 hours |
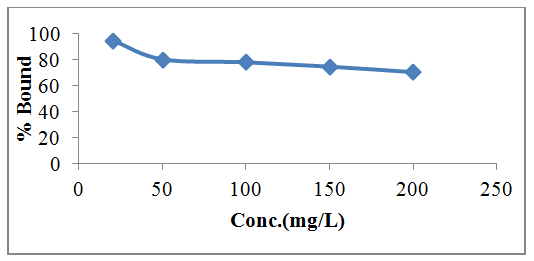 | Figure 3. Effect of different initial lead concentration on the percentage removal of Pb(II) from a 100ml aqueous solution containing 0.5g of the watermelon seed for 6 houra |
3.3. Effect of Adsorbent Dose
- Varying adsorbent dosage of 0.2 to 1.0 g were used to test for the effect of adsorbent dose on the biosorption process keeping the initial lead concentration at 100ml aqueous solution, while all other parameters were kept constant. It was observed as shown in Figure 4 that, when the adsorbent dose was increased from 0.2 g to 1.0 g, the percentage adsorption generally increased from 62.5% to 94.86%, but the amount adsorbed per unit mass of adsorbent decreased considerably. The fast increase in percentage uptake of lead at low dosage might be due to a larger external surface area available for the adsorption of the lead. The increase in the adsorption percentage or decrease in unit adsorption with increased dose of the biosorbent is due to the increase in active sites on the adsorbent and thus making easier penetration of the metal ions to the adsorption sites. When the adsorbent added is beyond 1.0 g, the decrease in lead ion adsorption is not very prominent which is perhaps due to the formation of adsorbent agglomerates reducing available surface area and blocking some of the adsorption sites. Maximum adsorption capacity of lead ions was observed with adsorbent dose of 1.0 g with adsorption capacity of 4.74 mg·g−1 and thereafter, a slow increase in the percentage removal was seen reaching a constant value with respect to adsorbent dosage.
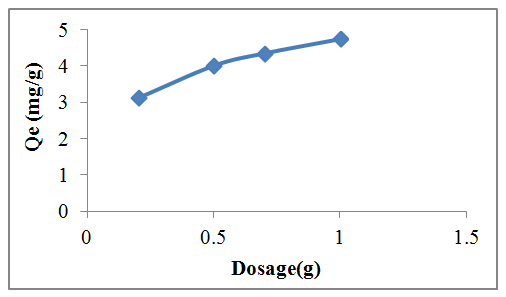 | Figure 4. Effect of adsorbent dose on the percentage removal of Pb(II) removal |
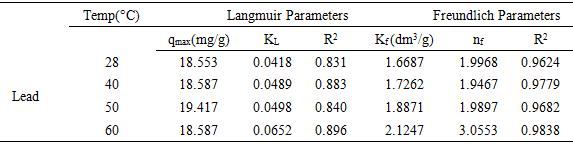
3.4. Effect of Temperature
- Effect of temperature on the adsorption of lead were studied at different concentrations. The concentrations were 20 mg/l, 50 mg/l, 100 mg/l, 150 mg/l and 200 mg/l at 28°C, 40°C, 50°C and 60°C. The observed increase in the adsorption of lead on watermelon seed powder with increase in temperature is indicative of the fact that the adsorption process is endothermic in nature. As shown in Figure 5, the increase in temperature of the system affects solubility and particularly the chemical potential of the adsorbate lead which is known to be a controlling factor in the process of adsorption. It has been reported earlier that if the solubility of the adsorbate increases and the chemical potential increases with rise in temperature, both these effects work in the same direction, thereby resulting in an increased adsorption [1].
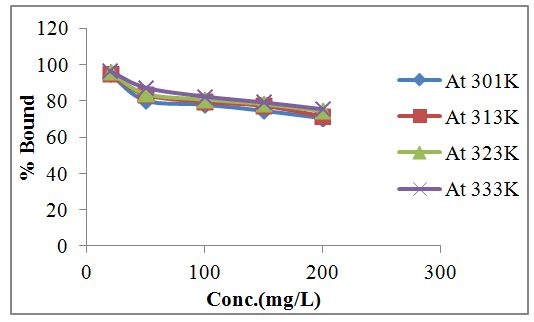 | Figure 5. Effect of temperature on Adsorption of Lead |
3.5. Kinetic Modeling of Biosorption
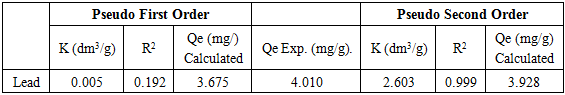
- Mass transfers occur within the boundary layer around the biosorbent and proceed in the liquid-filled pores or along the walls of the pores of biosorbent, which are called external and internal mass transfers, respectively. Typical kinetic models normally consider both the external and internal mass transfers. Examples of these models are film-pore diffusion, film-surface diffusion, pore diffusion, surface diffusion, and combined pore and surface diffusion models. These models involve complicated mathematical computations in order to obtain the related diffusion coeffcients of the models. Furthermore, the mass transfers of adsorption or biosorption often involve many controlling mechanisms, of which the individual contribution may not be clearly recognized, at the same time during the course to approach adsorption equilibria [7]. Therefore, for the simplicity and practical use of engineering applications, global kinetic expressions such as the Lagergren pseudo-first-order [19], pseudo-second- order [15] rate equations were adopted and applied to the biosorption data in order to analyze the rate of biosorption and possible adsorption mechanism of lead onto modified modified watermelon seed. The result of pseudo-first order was discarded because of lack of fit.
3.5.1. The Pseudo-Second-Order Kinetics Model
- The pseudo-second-order kinetics equation is expressed as Equation (4) according to [15]
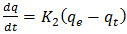 | (4) |
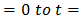
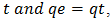 the integrated form of equation becomes:
the integrated form of equation becomes: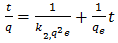 | (5) |
|
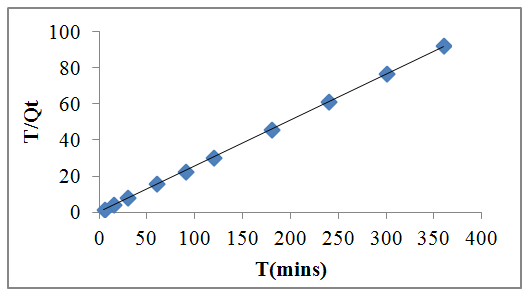 | Figure 6. Plot of second order kinetic model |
3.6. Adsorption Isotherm of Watermelon Seed
- Adsorption data are usually described by several adsorption isotherms, but not limited to Langmuir, Freundlich, Sips and Toth. The adsorption of lead ions using watermelon seed powder biosorbent was interpreted with Langmuir and Freundlich isotherm models and the certain assertions were made. Also, the reported implications of Sips and Toth isotherms were briefly discussed.
3.6.1. Langmuir Isotherm Model
- The Langmuir model is based on the assumption that the maximum adsorption occurs when a saturated monolayer of solute molecules is present on the adsorbent surface, the energy of adsorption is constant and there is no migration of adsorbate molecules on the surface plane [30]. It is represented linearly by the following equation 6
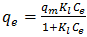 | (6) |
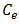 (mg/L) and
(mg/L) and 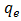 (mg/g) are the concentration of Pb(II) solution (mg/L) and the amount of adsorbed lead at equilibrium, respectively.
(mg/g) are the concentration of Pb(II) solution (mg/L) and the amount of adsorbed lead at equilibrium, respectively. 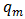 and
and 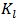 are Langmuir constants related to sorption capacity (mg/g) and the energy of sorption related to the heat of adsorption (L/mg) respectively.The essential characteristics of the Langmuir isotherm used to predict the favourability of the adsorption system can be expressed in terms of a dimensionless equilibrium parameter RL.An essential factor in the Langmuir isotherm is the separation factor RL
are Langmuir constants related to sorption capacity (mg/g) and the energy of sorption related to the heat of adsorption (L/mg) respectively.The essential characteristics of the Langmuir isotherm used to predict the favourability of the adsorption system can be expressed in terms of a dimensionless equilibrium parameter RL.An essential factor in the Langmuir isotherm is the separation factor RL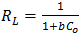 | (7) |
3.6.2. Freundlich Isotherm Model
- The empirical Freundlich isotherm is based on the equilibrium relationship between heterogeneous surfaces. This isotherm is derived from the assumption that the adsorption sites are distributed exponentially with respect to the heat of adsorption. The logarithmic linear form of Freundlich isotherm is represented in equation 8
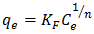 | (8) |
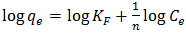 | (9) |
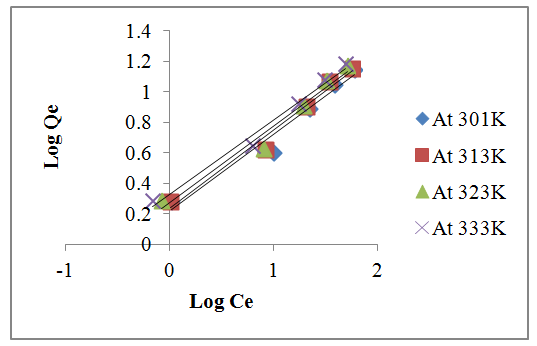 | Figure 7. Plot of Freundlich isotherm for lead |
|
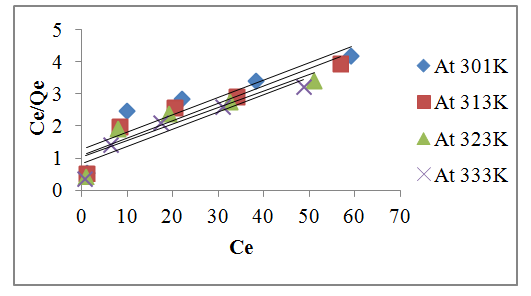 | Figure 8. Plot of Langmuir isotherm for lead at different temperature |
3.6.3. Sips Isotherm Model
- Sips isotherm is a combination of the Langmuir and Freundlich isotherms and it is given the general expression in Equation 10
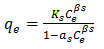 | (10) |
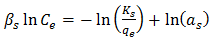 | (11) |
3.6.4. Toth Isotherm Model
- The Toth isotherm is another empirical modification of the Langmuir equation with the objective of minimizing the error between experimental data and predicted value of equilibrium data [34]. This model is most useful in describing heterogeneous adsorption systems which satisfy both low and high end boundary of adsorbate concentration [37]. The Toth isotherm model is expressed in Equation 12 as
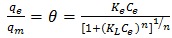 | (12) |
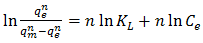 | (13) |
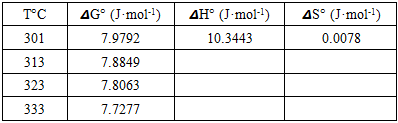
3.7. Thermodynamics Studies of Biosorption
- Thermodynamic parameters such as change in Gibb’s free energy ΔG°, standard enthalpy, ΔH° and standard entropy, ΔS° were determined using the equations 14 and 15:
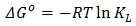 | (14) |
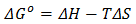 | (15) |
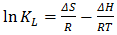 | (16) |
|
4. Conclusions
- The biosorption experiment carried out using watermelon seed was found suitable for the adsorption of Pb(II). The operational parameter (Adsorbent dose, temperature, initial metal ion concentration, time and pH) had varied effects on the percentage Pb(II) removal. The results of pseudo second order kinetic model for the adsorption of lead showed straight line graph which starts from the origin. The value 1/n greater than 1 is indicative of cooperative adsorption and a plot of log qe versus Ce at different temperatures indicates conformity with Freundlich isotherm. The high values of R2 for lead at 28, 40, 50 and 60°C were found to be 0.96, 0.98, 0.97 and 0.99 respectively. Thermodynamic parameters obtained were 𝞓G° (7.9792J/mol), 𝞓H° (10.3443 J/mol), S° (0.0078 J/mol·K). Watermelon seed has viable characteristics for preparing biosorbents.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML