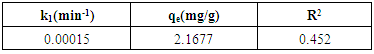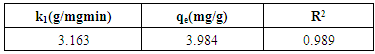-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2019; 9(1): 1-7
doi:10.5923/j.ajee.20190901.01

Equilibrium, Kinetics and Thermodynamic Studies of Adsorption of Cadmium(II) Ions unto Activated Carbon from African Elemi Seeds
Idris Aminu, Muhammad S. Sulaiman
Pure and Industrial Chemistry Department, Bayero University, Kano, Nigeria
Correspondence to: Idris Aminu, Pure and Industrial Chemistry Department, Bayero University, Kano, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The need to control our environment from industrial waste water pollution has ignited interest in cost effective products to treat industrial effluents. Adsorption of cadmium (II) ions from wastewater using activated carbon from African elemi seeds was studied. Batch adsorption experiments were carried out to investigate the effect of pH, contact time, initial metal ion concentration, temperature and adsorbent dosage on the adsorption efficiency. Results showed that increasing all the parameters, with the exception of temperature, increases the adsorption capacity. The process followed the pseudo-second order kinetics model which showed chemical adsorption. The experimental data was fitted well by the Freundlich isotherm indicating adsorption on heterogeneous surface. Thermodynamic data (negative ΔG, negative ΔH and positive ΔS) showed that the process was feasible, spontaneous, and exothermic with an increase in the randomness at the solid/solution interface. The study revealed that activated carbon from African elemi seeds could be used as a low cost adsorbent for removal of cadmium (II) ions from wastewater.
Keywords: Adsorption, Kinetics, Thermodynamics, Cadmium, Activated carbon
Cite this paper: Idris Aminu, Muhammad S. Sulaiman, Equilibrium, Kinetics and Thermodynamic Studies of Adsorption of Cadmium(II) Ions unto Activated Carbon from African Elemi Seeds, American Journal of Environmental Engineering, Vol. 9 No. 1, 2019, pp. 1-7. doi: 10.5923/j.ajee.20190901.01.
Article Outline
1. Introduction
- Industrial activities such as blacksmithing, mining, dyeing, tanning, metal plating, battery manufacture, petroleum refining, paint manufacture, printing etc., generate wastewater that is contaminated with heavy metals such as Cadmium, Zinc, Copper, Nickel, Lead, Mercury and Chromium (Kadirvelu et al., 2001; Adelaja, et al., 2011). These heavy metals are toxic, stable, non-biodegradable and when they contaminate water they become a serious threat to human health (Boudrahem, et al., 2011). Their accumulation in tissues of living organisms can cause diseases and disorders which necessitates their removal from wastewater before discharge. Cadmium is produced mainly from nickel-cadmium batteries, metal plating and plastic industries (Shotyk et al., 2005). It is extremely toxic even at low concentrations (Wang et al., 2011). The EU sets the maximum acceptable limit of cadmium in drinking water as 5 μg L-1 (Boudrahem et al., 2011).Various techniques such as adsorption, ion exchange, chemical precipitation, electro-dialysis, reverse osmosis, coagulation-flocculation, floatation and membrane processes have been used to treat industrial wastewaters (Mukesh and Lokendra Singh, 2013). Among these techniques, adsorption is superior in simplicity of design, highly efficient and is easier to operate which make it more favourable (Suteu and Bilba, 2005).The need to control our environment from industrial waste water pollution has ignited interest in cost effective products to treat industrial effluents. Biosorbents prepared from agricultural wastes and by-products have been widely studied because they are inexpensive, abundant in nature and require little processing (Wan Ngah and Hanafiah, 2008). Biosorption using renewable agricultural wastes offers a promising potential alternative to conventional technologies for treatment of industrial effluents. Adsorption studies have been carried out using plant residues such as grape stalk wastes (Villaescusa et al., 2004), moringa oleifera (Aminu et al., 2014), coffee wastes (Kyzas, 2012), degreased coffee beans (Kaikake, 2007), maize leaf (Babarinde et al., 2006), rice husk ash and neem bark (Bhattacharya et al., 2006), etc. However, the use of these plant residues for adsorption has its own problems: it has low adsorption capacity and increases the Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD) and Total Oxygen Demand (TOD) of the water (Wan and Hanafiah, 2008). One of the most effective and widely used adsorbents for treatment of water and wastewater is activated carbon, which is an amorphous form of carbon that is specially treated to produce a very large surface area. This structure provides activated carbon with the ability to adsorb gases and vapours from gases, and dissolved or dispersed substances from liquids (Othmer, 1979). It is the most widely used adsorbent since most of its chemical (e.g. surface groups) and physical properties (e.g. pore size distribution and surface area) can be tuned according to what is needed.In developing countries, the use of commercial activated carbon is often too costly; therefore, emphasis is made on the preparation of the AC from locally available materials for use as adsorbents. A number of studies have been carried out on the preparation of AC from agricultural and industrial by-products for use as adsorbent (Ahmedna et al., 2004; Aloko and Adebayo, 2007; Aygun et al., 2003; Bansode et al., 2002). There is a constant need to investigate the potentials of various materials as activated carbon precursors depending on their cost and availability (Misihairabgwi et al., 2014).In the present work, African Elemi (Canarium schweinfurthii) seeds were subjected to chemical activation method to produce activated carbon that was used for removal of Cd(II) ions from synthetic wastewater. The aim was to investigate the capacity of activated African Elemi seed for adsorbing Cd(II) ions. The effects of agitation time, adsorbent dosage, pH, temperature, initial ion concentration, and contact time on the adsorption capacity were studied. Kinetics and thermodynamics studies were carried out while adsorption isotherms were used to model the adsorption process.
2. Materials and Methods
2.1. Adsorbent
- The African Elemi Seeds were collected randomly within Jos, Plateau State, Nigeria. A sample of the seeds was thoroughly washed with de-ionized water to remove dirt and other particulate matter. The fresh sample was dried in an oven for six hours, at temperature of 100°C and grounded using mortar and pestle. It was then soaked with n-hexane for three days to remove the fats in the seeds after which, it was thoroughly washed again with de-ionized water, then dried and weighed. Impregnation was done with 0.1M HNO3 for about six hours and then filtered. The sample was carbonized in an electric muffle furnace for six hours at temperature of 300°C in an inert atmosphere and then cooled in the desiccators. The activated carbon was washed with de-ionized water, dried and weighed. The sample was again subjected to a further treatment by grinding and sieving with a 250μm sieve to get a very fine powder then stored in an air tight container.
2.2. Characterization of the Activated Carbon
- The activated carbon yield, ash content, bulk density and pH were determined using the method of Ahmedna (1997). The major functional groups in the adsorbent were determined by Fourier transform infrared spectrophotometer (FTIR-8400S).
2.3. Batch Adsorption Experiments
- Batch adsorption experiments were performed to determine the effects of temperature, initial metal ion concentration, contact time, pH and adsorbent dosage on the adsorption of Cd(II) ions from solution. For each experiment, 50 mL of synthetic wastewater containing a known concentration of Cd(II) ions was shaken with a calculated amount of the activated carbon in a flask at a known temperature and for a known duration. The pH of working solutions was adjusted to the desired values by the addition of either 0.1M HCl or 0.1M NaOH solution. In each case, after equilibrium, the mixture was filtered using Whatman No.1 filter paper and the concentration of Cd(II) ions remaining in the solution was determined using atomic absorption spectrophotometer (Perkin Elmer; Analyst 200).The amount of metal ion adsorbed by the adsorbent and the percentage removal of the metal ion were calculated by using the following equation (Al-Anber and Al-Anber, 2008):
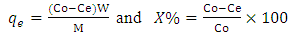 Where qe is the amount of Cd(II) ions adsorbed on the activated carbon per gram adsorbent (mg/g); Co is the initial concentration of Cd(II) ions in solution (mg/L); Ce is the concentration of Cd(II) ion at equilibrium with the solid phase (mg/L); W is the volume of metal ion solution (L); M is the mass of the adsorbent (g) and X% is the percentage of metal ions removed.
Where qe is the amount of Cd(II) ions adsorbed on the activated carbon per gram adsorbent (mg/g); Co is the initial concentration of Cd(II) ions in solution (mg/L); Ce is the concentration of Cd(II) ion at equilibrium with the solid phase (mg/L); W is the volume of metal ion solution (L); M is the mass of the adsorbent (g) and X% is the percentage of metal ions removed.3. Results and Discussion
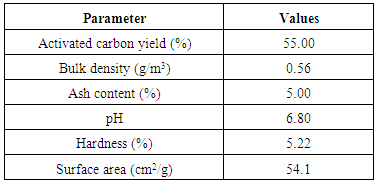
3.1. Physicochemical Analysis of the Activated Carbon
- The results of the physicochemical analysis of the activated carbon are presented in Table 1. All the values fall within the reported range in literatures (Long and Criscione, 2013; Bassey et al., 2015).
|
3.2. Functional Groups
- The FTIR spectra are shown in Fig. 1 while a summary of the peaks and their possible assignments are given in Table 2. Result of peaks displayed by FTIR due to the presence of some functional groups on the surface of activated carbon powder is translated as follows:
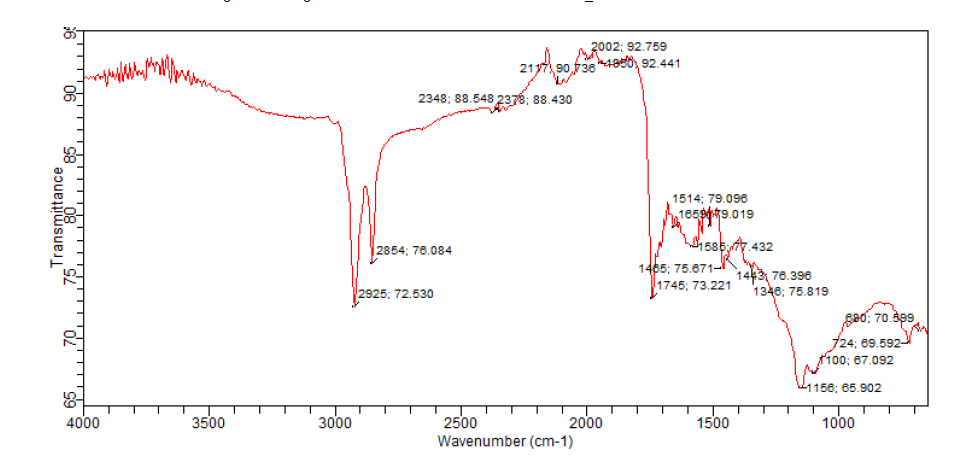 | Figure 1. FTIR spectra of the activated carbon |
|
3.3. Effect of pH
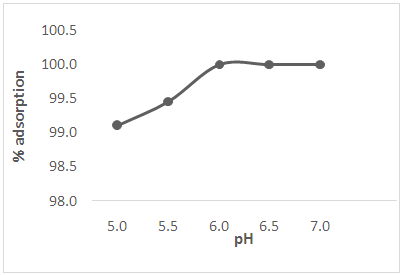 | Figure 2. Effect of pH on the adsorption of Cd(II) ions from solution |
3.4. Effect of Contact Time
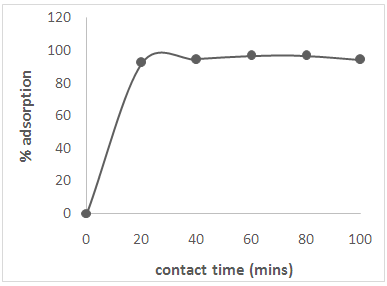 | Figure 3. Effect of contact time on the adsorption of Cd(II) ions from solution |
3.5. Effect of Initial Metal Ion Concentration
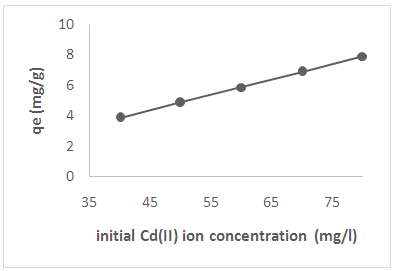 | Figure 4. Effect of initial metal ion concentration on adsorption of Cd(II) ions from solution |
3.6. Effect of Temperature
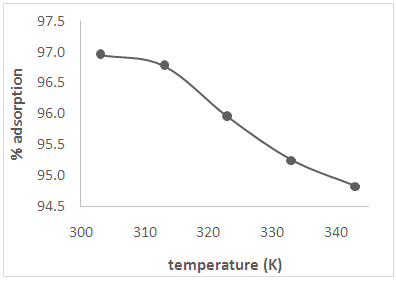 | Figure 5. Effect of temperature on the adsorption of Cd(II) ions from solution |
3.7. Effect of Adsorbent Dosage
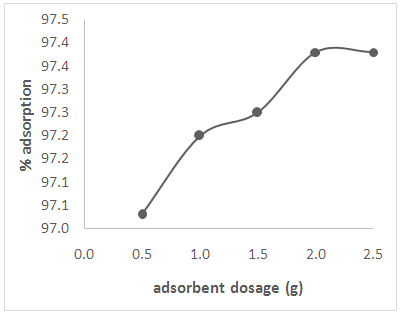 | Figure 6. Effect of adsorbent dosage on the adsorption of Cd(II) ions from solution |
3.8. Adsorption Kinetics
- Kinetics studies are important in the design and operation of adsorption systems (Akpomie et al., 2013). In analyzing the controlling mechanism for the adsorption of Cd(II) ions onto the activated carbon, pseudo-first order and pseudo-second order kinetic models were used. The kinetic parameters for pseudo-first order and pseudo-second order models are shown in Tables 3 and 4 respectively. Low correlation coefficient of the pseudo-first order model shows that the reaction is not elementary and the adsorption is not exclusively for one site per ion. The adsorption of Cd(II) ions onto the activated carbon can best be described by the pseudo-second order model due to larger correlation coefficient. This shows that the adsorption of Cd(II) ions by the activated carbon follows the second order mechanism where rate of occupation of adsorption sites is proportional to the square of the number of unoccupied sites (Adekola et al., 2014). This implies that the rate limiting step could be chemisorption promoted by either valency forces or covalent forces (Boudrahem et al., 2011).
|
|
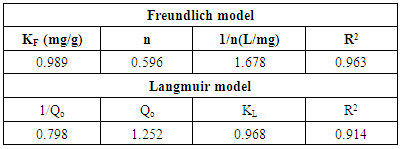
3.9. Adsorption Isotherms
- Adsorption isotherms show the relationship between the amount of adsorbate on the adsorbent and its equilibrium concentration in solution (Akpomie et al., 2013). Important information on the mechanism of the adsorption and the surface properties of the adsorbents are provided by the adsorption isotherms. The adsorption experiments in this work were described using the Langmuir and Freundlich isotherm models.The Langmuir isotherm is based on the assumption that each adsorption site can accumulate only one molecule with no interactions between the sites, the adsorption surface is homogeneous and only a monolayer is formed on the adsorbent. It is expressed mathematically as:
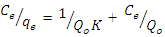 where Ce is the equilibrium concentration (mg L−1), qe the amount adsorbed at equilibrium (mg g−1), Q0 is the monolayer adsorption capacity of the adsorbent and K is the Langmuir constant related to adsorption capacity and energy of adsorption. The linear plot of Ce/qe versus Ce gives a straight line and Qo and K are determined from the slope and intercept of the plot.The Freundlich isotherm model is based on adsorption on heterogeneous surface with independent sites where there is an exponential decrease in the sorption site energy distribution (Boudrahem et al., 2011). The linearized form of the equation is given as:
where Ce is the equilibrium concentration (mg L−1), qe the amount adsorbed at equilibrium (mg g−1), Q0 is the monolayer adsorption capacity of the adsorbent and K is the Langmuir constant related to adsorption capacity and energy of adsorption. The linear plot of Ce/qe versus Ce gives a straight line and Qo and K are determined from the slope and intercept of the plot.The Freundlich isotherm model is based on adsorption on heterogeneous surface with independent sites where there is an exponential decrease in the sorption site energy distribution (Boudrahem et al., 2011). The linearized form of the equation is given as: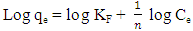 where KF (mg/g)(mg/L)1/n and n are the Freundlich constants related to adsorption capacity and adsorption intensity, respectively. A plot of log qe against log Ce gives a straight line with an intercept and slope KF and 1/n respectively.Table 5 presents the isotherm parameters for both Freundlich and Langmuir models. It was observed that the Freundlich model describes the experimental data better than the Langmuir model because of high correlation coefficient. This shows that the surface of the activated carbon is heterogeneous with independent binding sites. More realistic description for adsorption on organic matter is given by the Freundlich model because it accounts for different binding sites (Soltani et al., 2009).
where KF (mg/g)(mg/L)1/n and n are the Freundlich constants related to adsorption capacity and adsorption intensity, respectively. A plot of log qe against log Ce gives a straight line with an intercept and slope KF and 1/n respectively.Table 5 presents the isotherm parameters for both Freundlich and Langmuir models. It was observed that the Freundlich model describes the experimental data better than the Langmuir model because of high correlation coefficient. This shows that the surface of the activated carbon is heterogeneous with independent binding sites. More realistic description for adsorption on organic matter is given by the Freundlich model because it accounts for different binding sites (Soltani et al., 2009).
|
3.10. Thermodynamic Studies
- Thermodynamic studies show the feasibility and spontaneity of the adsorption process and the structural changes due to metal ion binding. Thermodynamic parameters were investigated for the adsorption of Cd(II) ions onto the activated carbon within the range 303 – 343K while keeping other parameters constant. The free energy change is given by the following expressions (Liu and Liu, 2008)ΔGo = - RT ln Kin which T is absolute temperature and R is the gas constant.ln K = - (ΔHo/RT) + (ΔSo/R)The values of standard enthalpy change (ΔHo) and standard entropy change (ΔSo) were obtained from the slope and intercept respectively, of the graph of ln K against 1/T (K-1). The thermodynamic parameters are shown in Table 6. The value of the correlation coefficient (0.968) shows good agreement between the free energy change and the temperature.
|
4. Conclusions
- African elemi seeds have been successfully converted to activated carbon and used as a low cost adsorbent for Cd(II) ions removal from wastewater. Based on the experimental results obtained, the following conclusions were drawn: v FTIR spectra showed the presence of some functional groups responsible for binding of the metal onto the activated carbon.v The contact time, pH, initial metal ions concentration, adsorbent dosage and temperature had a remarkable effect on the metal adsorption. v The adsorption process of the metal ions was best described by a pseudo-second order.v The Freundlich isotherm model fitted well the experimental data indicating a heterogeneous surface with independent binding sites. v The thermodynamics parameters showed that the adsorption of Cd(II) ions by the activated carbon was spontaneous and exothermic, with an increase in the randomness at the solid/solution interface during the adsorption process.v Activated carbon from African elemi seeds could be used as a low cost adsorbent for removal of cadmium (II) ions from wastewater.
ACKNOWLEDGEMENTS
- The authors wish to thank Bayero University Kano for providing the materials and the laboratory for the work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML