-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2018; 8(2): 25-35
doi:10.5923/j.ajee.20180802.02

Combined Clay Adsorption-Coagulation Process for the Removal of Some Heavy Metals from Water and Wastewater
Ababu T. Tiruneh1, Tesfamariam Y. Debessai2, Gabriel C. Bwembya2, Stanley J. Nkambule1
1University of Swaziland, Department of Environmental Health Science, Mbabane, Swaziland
2University of Swaziland, Department of Chemistry, Kwaluseni, Swaziland
Correspondence to: Ababu T. Tiruneh, University of Swaziland, Department of Environmental Health Science, Mbabane, Swaziland.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Heavy metals pollutions from wastewater discharges present an increasing concern for health, eco-system and the environment due to their persistence and bio accumulation via the food chain. Heavy metals in excess concentration cause problems of toxicity and growth inhibition to living things. While the cation exchange capacity of clay with respect to heavy metal removal is well documented, a two-step clay-coagulation combined process is studied in this research in which an excess clay addition in powder form to wastewater is followed by coagulation with aluminum sulphate to further remove heavy metal by synergy with the clay adsorption and to settle the clay adsorbent through flocculation and settlement. High percentage removal of heavy metals ranging from 96-99% has been observed. The saturated adsorption is adequately modelled by Freundlich as well as Langmuir isotherm models for the excess clay range studied. The clay-coagulation combined treatment technology can also be adapted to heavy metal removal in water treatment applications in which coagulation is often used for the removal of the colloidal portion of suspended solids.
Keywords: Heavy metal removal, Clay adsorption, Wastewater treatment, Pollution, Coagulation, Freundlich isotherm, Environmental protection
Cite this paper: Ababu T. Tiruneh, Tesfamariam Y. Debessai, Gabriel C. Bwembya, Stanley J. Nkambule, Combined Clay Adsorption-Coagulation Process for the Removal of Some Heavy Metals from Water and Wastewater, American Journal of Environmental Engineering, Vol. 8 No. 2, 2018, pp. 25-35. doi: 10.5923/j.ajee.20180802.02.
Article Outline
1. Introduction
- The presence of heavy metals in the natural environment including air, water, soil and plants is becoming an increasing health and environmental concern owing to the wide range of anthropogenic sources of heavy metals polluting the environment with growing industrialization and extensive use of chemicals (Srinivasan, 2011; Kinder, 1997, Yakun et al., 2014). Wastewater effluent streams from many industries display wide range of heavy metals in varying concentrations (Missana and Garcı, 2007). Heavy metals can directly enter the human body through the food chain causing serious health danger if present in excess concentration beyond permissible limits (Lin et al., 2000). Heavy metals are persistent and can bio accumulate in organisms disrupting the metabolic functions and vital organs in humans and animals (Tchounwou et al., 2012). They can cause wide range of illnesses including renal and organ damage, neurological dysfunction, heart disease, allergy, asthma, respiratory problems, cancer as well as cardio vascular effects (Barbooti, 2015). Heavy metals are present in water either in the form of free ions or as complexes with organic and inorganic ligands (Alleoni et al., 2003). Various types of treatment technologies have been adopted in the past for heavy metal pollutant removal. These include liquid membrane (Gürel et al., 2005), bagasse (Gupta et al., 1998), clay (Al-Jlil and Alsewailem, 2009), ion exchange (Rengaraj et al., 2003; Roque-Malherbe et al., 2007), precipitation ( Esmaeili et al., 2005) and biosurfacant (Kim and Vipulanandan, 1997).Adsorption is a surface phenomenon, a physico-chemical separation process whereby the adsorbate material is transferred from the bulk liquid phase to the adsorbent solid surface. Removal of heavy metals through adsorption is well studied and is claimed to have several advantages over other methods of removal such as precipitation, ion exchange or membrane based processes. Such advantages include low cost and easy availability especially with natural clay being abundantly available worldwide. Such adsorbents provide cost effective alternatives to conventional treatment technologies (Srinivasan, 2011; Sanchez et al., 2002). Other advantages include simplicity of operation and high removal efficiency (Oswald et al., 2008). Compared with several other adsorbents available for heavy metal removal, adsorbents of natural clay material offer better adsorption capacity while being low cost and easily available (Srinivasan, 2011). Adsorbents such as activated carbon are reportedly 20 times more expensive than clay (Babel and Kurniawan, 2003; Virta, 1996). Clay minerals are alumino sillicates groups with sizes falling in the colloid fraction of soils with particle sizes smaller than 0.002 mm (Pinnavaia, 1983; Oloafe et al., 2015). They are classified into: montmorillonite, smectite, kaolinite, illite, and chlorite. Montmorillonite, kaolinite, and illite are commonly used adsorbents owing to their greater surface area and structural rigidity (The Clay Mineral Group, 2011; Lin and Juang, 2002; Krishna et al., 2000; Bailey, 1999). Clay soils are known for their high cation exchange capacity that makes them preferred adsorbent materials (Cadena et al., 1990). The net negative charge present on the clay structure is because of substitution of silicon ion (Si4+) by aluminum ion (Al3+) that gives rise to Bronsted acidity formed on the surface by dissociation of water molecules. This acidity enhances the adsorption capacity (Tanabe, 1981). Lewis acidity in turn arises because of exposed trivalent Al3+ ions following structural rupture of the Si-O-Al bonds by dehydroxylation of some Bronsted acid sites (van Olphen, 1977). Clay adsorbents have demonstrated excellent removal of heavy metals or metalloids including As, Cd, Cr, Co, Cu, Fe, Pb, Mn, Ni and Zn from water. Montimorrlinite have high adsorption capacity compared with kaolinite and illite (Bhattacharyya and Gupta, 2008). Lead was removed almost 100% and chromium 86% according to Barbooti (2015) indicating lead is removed better than chromium. High percentage removal of heavy metal is aided by excess clay addition as much 12.5 g/L of wastewater using montmorillonite clay (Barbooti, 2015). Arsenic removal has been studied with membranes formed from montmorillonite, kaolinite, and illite operated through pumping (Fang et al., 2006). Removal by rejection as high as 90% of arsenic in the feed stream has been reported. Nanostructured polymer-clay composites, in which small amount of nano-clay particles are added to enhance mechanical, thermal, dimensional and barrier performance properties, have been used as sorbents for wide range of pollutants in ionic form, organic pollutants, herbicides, atrazines and others (Churchman, 2002; Breen, 1999; Radian and Mishael, 2008; Zadaka et al., 2008). Adsorption parameters such as pH, adsorbent dosage, adsorption kinetics, mixing time, ionic strength of the wastewater and temperature are important parameters as they exert influence on the adsorption process. Higher pH favours greater removal of heavy metals with increasing acidity facilitating desorption process because of increased competition from excess hydrogen ions present at low pH. When the pH increases from the acid to neutral range, the extent of adsorption increases. On the other hand, as the pH increases further to the basic range the mode of removal shifts from adsorption to removal by precipitation (Heba and Mikhail, 2014). Beyond pH 8, the dominant removal mechanism of heavy metals is precipitation, electrostatic attraction and surface complexation (Bellir et al., 2013). In a batch equilibrium study made by Sajidu et al. (2006), complete removal of chromium was obtained from pH 3 to 5. Zinc was removed completely above pH 7, copper above pH 4, cadmium between pH 6 and 9 and lead above pH 7.7. However, no effective removal of AsO43− anion was observed. Adsorbent dosage directly influences the adsorption efficiency since with greater amount of adsorbent used the number of active sites available for adsorption increases thereby increasing the adsorption efficiency. According to Bellir et al (2013), adsorbent dosage of 0.5 g/l was associated with 85% removal of heavy metals. Increasing the dosage to 0.8 g/l increased the percentage removal to 92%. The kinetics of removal appears to be second order overall (Barbooti, 2015). This has also been confirmed by Bellir et al (2013) in their research on adsorption of cadmium and zinc on clay bentonite. The pseudo first order model gave poor data fit with poor correlation coefficient whereas the pseudo second order kinetics gave the best fit with very high correlation coefficient value.Mixing time required for adsorption is influenced by the different phases of adsorption processes involving bulk diffusion, film diffusion, intra particle diffusion and adsorption onto the interior active sites. A mixing (shaking) time between 30 and 60 minutes will ensure maximum adsorption reaching equilibrium concentrations after overcoming the different resistances (Bellir et al., 2013). The ionic strength of the wastewater influences the extent of adsorption of heavy metals on to clay since solutions with higher ionic strength create competition of these ions with heavy metals for adsorption. Therefore, the higher the ionic strength of the wastewater, the lesser will be the extent of heavy metal adsorption (Ihaddadene, et al., 2016). Temperature directly influences the adsorption process, as adsorption is often endothermic reaction whereby the rate of adsorption increases with increase in temperature. According to the experiments of heavy metal adsorption on bentonite clay by Aljlil and Fares (2014), increasing the temperature of reaction resulted in positive enthalpy change confirming that the adsorption is an endothermic process. In addition, the Gibbs free energy change decreased with increasing temperature demonstrating further that adsorption is favoured at higher temperature. The concept of combined-clay coagulation for the removal of heavy metals adopted in this research is thought to derive maximum benefit from the synergy of clay adsorption capacity with the coagulation potential of coagulants while achieving easier removal of adsorbent through flocculation. The synergy between the clay and the coagulant may be attained further through increased mobility of heavy metals from the diffuse layer to the clay during the flocculation process because of the decrease of the zeta potential. According to Almeida et al. (2012), the formation of electrical double layer with the fixed and diffuse layers decreases the adsorptive capacity of the clay adsorbent because of the existence of metals in the diffuse layer that moves eventually with the liquid during the separation process. Tushar et al. (2002) also reasoned that leakage of heavy metals occurs because of transport of colloidal size particles with the flow especially where the solid-liquid separation process is not efficient. Furthermore, the use of coagulation alone for heavy metals removal requires higher coagulant dosage in order to remove the metal by co-precipitation (Akbal, and Camci, 2010). In the proposed combined clay-coagulation setup used in this research, less coagulant dosage would be needed, as adsorption by the clay adsorbent would be the dominant mode of heavy metal removal.
2. Materials and Methods
- The method employed for heavy metal removal process in this research is based on a combination of clay addition into wastewater in a powder form followed by the process of coagulation for enhancing the removal of heavy metal through synergy with the clay adsorbent and for the removal of the clay adsorbent by flocculation and settlement. The addition of excess clay ensures a good percentage removal of heavy metal whereas the coagulation process makes it easier to separate the clay adsorbent from the wastewater in addition to decreasing the zeta potential and thereby enabling further adsorption of metals from the diffuse layer onto the clay adsorbent. Coagulation further removes the heavy metals by co-precipitation during the flocculation-precipitation stage. The addition of clay in powder form eliminates the pore resistance to adsorption as well as frictional resistance to flow that are normally present in packed adsorbent columns. Besides, coagulation removal of clay adsorbent is handier and efficient than removing clay through filtration process.Wastewater leachate samples were collected from the Mpolonjeni solid waste landfill site that is located in Mbabane City. Because of the limited heavy metal content of the leachate samples present during the time of collection, the method of standard addition was used in order to increase the heavy metal contents of the wastewater. The experimental procedure consisted of adding known concentrations of heavy metals to measured volumes of wastewater samples followed by addition of clay adsorbent in powder form. The final step was coagulation with aluminum sulphate for the removal of the clay adsorbent followed by flocculation and settlement. The detail procedure followed during the experiments is specified below.
2.1. Preparation of Heavy Metals for Addition into Samples
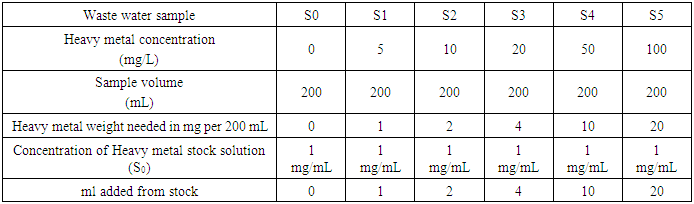
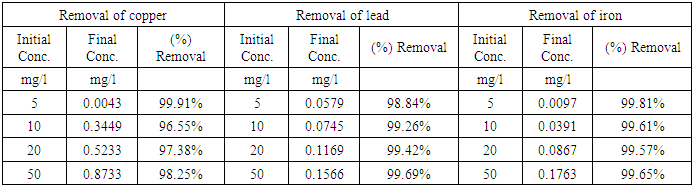
- Heavy metal standards to be added to the wastewater samples were prepared for each of the heavy metals of copper, iron and lead. Stock solutions of each heavy metal with concentration measuring 1000 mg/L were used which were analytical reagent grade metal standards with 1% nitric acid added into the stock solutions. The volumes of heavy metals added from the stock solution for each of the heavy metals to give different heavy metal concentrations are summarized in Table 1.
|
2.2. Preparation of Heavy Metal Standard Calibration Solutions for Atomic Absorption Spectrometer Measurement
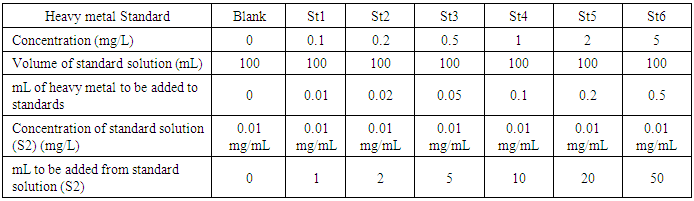
- Standards ranging between 0 and 5 mg/l were prepared since in this low range linearity of the standard calibration for the studied heavy metals is ensured by the atomic absorption spectrometer instrument. From the stock solution of heavy metals measuring 1000 mg/L, subsequent standard solutions (S1 and S2) were prepared to give concentrations respectively of 100 mg/L for S1 and 10 mg/L for S2 standards. Distilled-deionized water was used for the dilution water needed for preparing these standards. Suitable mL volumes were added from standard solution S2 to give the desired standard ranging between 0 (blank) and 5 mg/L. This procedure is summarized in Table 2.
|
2.3. Adsorbent Clay Material
- Red clay soil (Utisol) samples needed for experiment were collected from suitable locations in Mbabane City. These clay soils are abundantly available in Swaziland, their common use being for traditional pottery, ceramics and as building materials such as bricks. Utisols have varieties of clay minerals but the dominant one is kaolinite. It has been demonstrated (Talaat, 2011) that raw kaolin, rather than kaolin treated with acid washing, gives better performance in terms of heavy metal removal by adsorption. Untreated raw red clay samples have been demonstrated to have had higher cation exchange capacities due to the presence of organic material and iron oxides (Pare et al., 2012). In this experiment, therefore, no acid treatment has been applied to the red clay used for adsorption.The clay samples were ground and sieved to mesh size #100 and then dried at 120°C for 3 hours. The clay samples were weighed for addition into samples of wastewater in which heavy metals were present. Since the experiments were based on excess clay addition in order to test the feasibility of the combined clay-coagulation process, 20 g. of clay were added to each of the 200 mL of water samples with differing heavy metal concentrations.
2.4. Determination of Optimum Coagulant Dosage
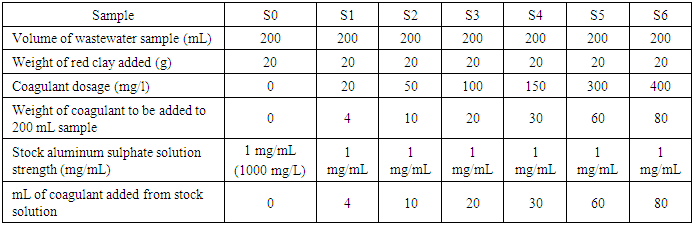
- The optimum amount of aluminum sulphate coagulant needed to settle the clay adsorbent was determined through jar test. First a stock solution of aluminum sulphate was prepared to give a concentration measuring 1000 mg/L. From this stock solution, different coagulant dosage volumes were added to each of the six beakers each of which contain the excess 20 g. red clay adsorbent in 200 mL of water. The coagulant addition was immediately followed by rapid mixing for one minute and subsequent flocculation through slow stirring for 15 minutes. The flocs formed after flocculation were then allowed to settle for 30 minutes. The turbidity of the settled supernatant was measured afterwards. Table 3 shows the procedure employed for coagulant addition in preparation for the jar test experiment.
|
2.5. Combined Clay-Coagulation Experiment
- The combined clay-coagulation experiment was conducted using the optimum coagulant dosage that was determined through the Jar test for a given weight of excess clay adsorbent used in the experiments. First 200 mL of the wastewater samples were added to each of the beakers used for the experiment. This was followed by the addition of heavy metals into the sample beakers to give concentrations ranging between 0 and 100 mg/L. Then after the excess clay adsorbent was added to each of the wastewater samples and the mixture was thoroughly mixed allowing at the same time sufficient time for adsorption to occur. Procedures such as centrifuging and filtration used to separate the heavy metal from the clay adsorbent were not necessary in this experimental setup as the coagulant readily removes the clay adsorbent in simpler coagulation process. Finally, the optimum aluminum sulphate coagulant dose determined from the jar test was added to each of the samples, which was accompanied by rapid mixing, flocculation ad settlement. The settled supernatant samples were decanted and taken for heavy metals determination using atomic absorption spectrometer. The heavy metal concentrations remaining in the supernatant following the combined adsorption-coagulation were determined using flame atomic absorption spectrometer, FAAS (AA-250 Varian). Air and acetylene were used for creating the gas flame. The instrument was calibrated in the linear range using standard series in the range between 0 and 5 mg/|L prepared for each of the heavy metals. This calibration was done for each of the heavy metals determination before measurements were taken. Where dilutions were needed for sample measurements that were out of the linear range, the needed dilutions were made and measurements recorded. The amount of heavy metal adsorbed (X) per unit mass of clay adsorbent used (M) was calculated using the following formula
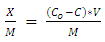 | (1) |
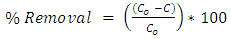 | (2) |
2.6. Adsorption Modeling
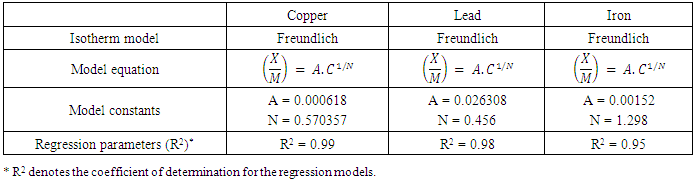
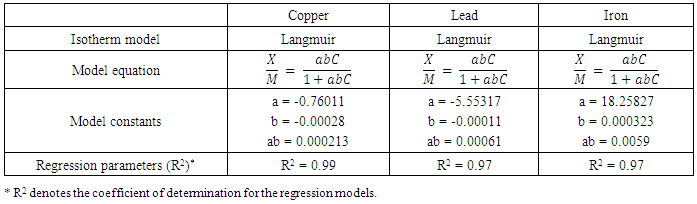
- Modeling of the adsorption process of heavy metals using clay adsorbents are often based on either Freundlich or Langmuir isotherm models. The Langmuir isotherm assumes uniform adsorption resulting in a saturated monolayer of adsorbate materials occupying the adsorption surface. It also assumes that there is no further interaction among the adsorbate materials (Kim et al., 2013; Foo and Hameed, 2010). The Freundlich isotherm by contrast is an empirical model whereby the adsorption is composed of heterogeneous processes with the stronger binding sites occupied first and with decreasing energy of adsorption as the adsorption progresses towards the weaker processes (Al-Shahrani, 2014). In many cases, the Freundlich isotherm gives best fit to the adsorption data (Burham and Sayed, 2016) rather than the Langmuir adsorption isotherm. Several researches carried out reported Freundlich isotherm model giving the best data fit confirming that the clay adsorption of heavy metals is a multi-layer sorption process (Glatstein and Francisca, 2015; Eren et al., 2010). In this research, both the Freundlich and Langmuir isotherms were fitted to the adsorption data to test their appropriateness to the combined clay-coagulation adsorption process. The Freundlich isotherm model is based on the equation:
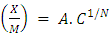 | (3) |
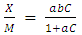 | (4) |
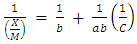 | (5) |
2.7. Initial Wastewater Quality Assessment of the Leachate Sample
- The initial wastewater quality of the leachate sample on which heavy metal standard addition was made were determined for a number of parameters that may influence the adsorption process. The parameters determined include conductivity, total dissolved solids, pH, suspended solids, COD, BOD, ammonia, colour, chloride, dissolved oxygen, turbidity and sodium. The standard operation procedures specified in the Standard Methods for the examination of water and wastewater were used for the determination of these water quality parameters.
3. Results and Discussion
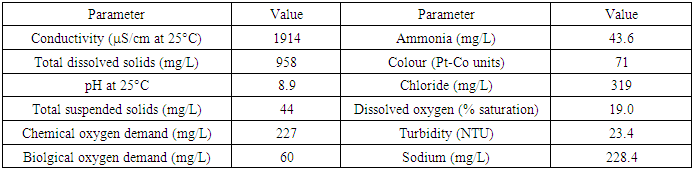
- The initial wastewater quality analysis of the leachate sample was carried out in order to determine the environmental variables under which the clay adsorption-coagulation takes place. It was mentioned in the introduction section of this paper that parameters such as ionic strength and pH affect the adsorption process. This paper is not aimed at varying these variables as the factors are well studied. For example higher pH is known to favour adsorption and precipitation of heavy metals. Greater ionic strength of the wastewater solution also affects heavy metal removal because of competitive adsorption by other cations present in the wastewater. Table 4 presents the result of the wastewater quality analysis of the leachate sample. It is clear that the leachate pH is high (8.9) which favours the heavy metal adsorption. On the other hand, the total dissolved solids of the wastewater is also high (958 mg/L) which to a certain extent reduces the extent of heavy metals removal because of competitive adsorption. Other parameters such as COD, BOD and ammonia are also included in the result shown in Table 4.
|
|
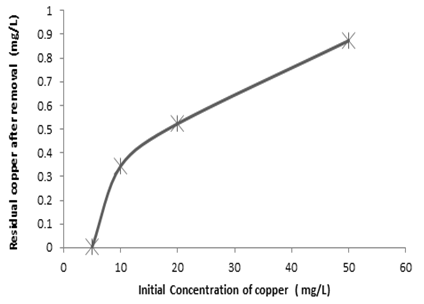 | Figure 1. Variation of residual concentration of heavy metals with initial heavy metal present for copper |
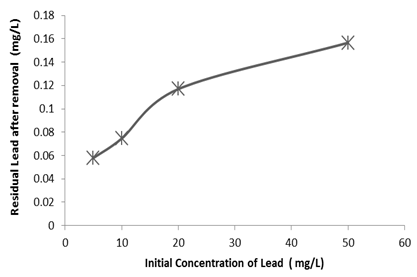 | Figure 2. Variation of residual concentration of heavy metals with initial heavy metal present for lead |
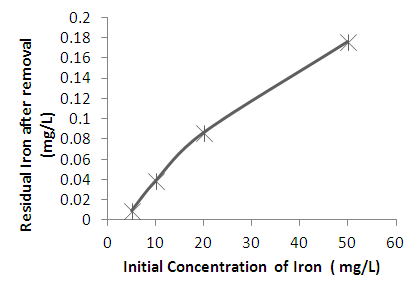 | Figure 3. Variation of residual concentration of heavy metals with initial heavy metal present for iron |
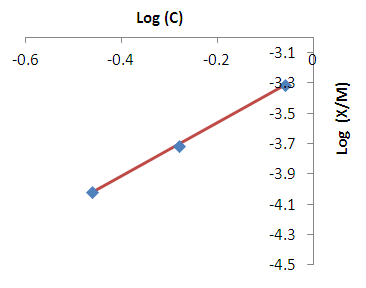 | Figure 4. Freundlich isotherm fit for the removal of copper |
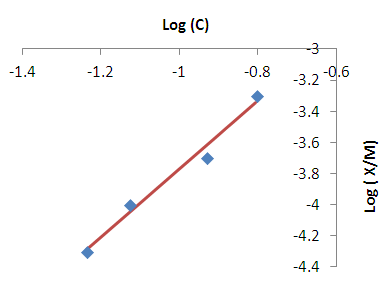 | Figure 5. Freundlich isotherm fit for the removal of Lead |
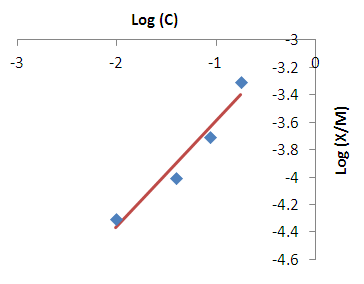 | Figure 6. Freundlich isotherm fit for the removal of iron |
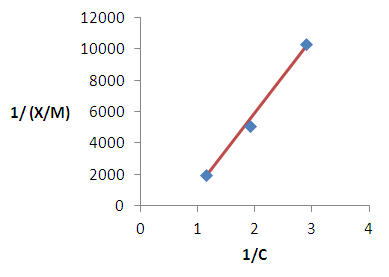 | Figure 7. Langmuir isotherm for the removal of copper |
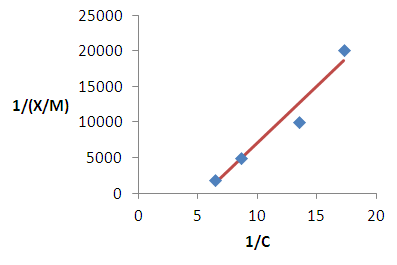 | Figure 8. Langmuir isotherm for the removal of lead |
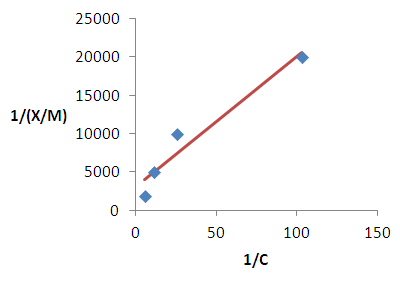 | Figure 9. Langmuir isotherm for the removal of iron |
|
|
4. Conclusions
- From the experimental results obtained, it is apparent that the combined excess clay-coagulation process gives high percentage removal for all the three heavy metals studied. The percentage removals range between 96% and 99%. The efficiency of removal for the red clay used in the experiment favors iron and to a certain extent lead at higher initial metal concentrations whereas at low concentrations removal of copper is favored. The clay-coagulation process significantly reduces the pore and film diffusion resistances that are normally present on adsorbent columns since the clay is applied in powder form and is eventually removed through coagulation. In addition, the coagulation step provides synergy with the clay adsorption by decreasing the zeta potential and allowing more heavy metals to be adsorbed from the diffuse layer. Removal by co precipitation occurs further following coagulation. The coagulation step, apart from enhancing the heavy metal removal by synergy with the clay adsorption, represents a simpler method for separating the adsorbent from the supernatant compared with other methods such as centrifuging and filtration where powder form is used for adsorption. Another bottleneck with the traditional clay adsorption is the low permeability of the clay adsorbent which is absent in this technological setup. The clay adsorbent is completely removed through the addition of optimum dose of the coagulant, which is used for the purpose of flocculating the clay adsorbent and separating it from the treated water by sedimentation or filtration. In addition, the combined clay-coagulation process may offer additional benefits through removal of other pollutants such as organic matter making it potentially useful for wide range of wastewater pollutants. The research setup can be used not only in wastewater treatment applications but also in conventional water treatment processes where coagulation is used. The simple modification of adding the clay adsorbent to such conventional treatment processes as combined treatment makes them capable of removing heavy metals that are otherwise difficult to remove through, conventional water treatment setups.
ACKNOWLEDGEMENTS
- The researchers would like to thank the Department of Chemistry of the University of Swaziland for providing access to laboratory facilities for heavy metal analysis using atomic absorption spectrometer.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML