-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2018; 8(2): 17-24
doi:10.5923/j.ajee.20180802.01

Pathogens Removal from Wastewater Using Sustainable Treatment Wetlands in Tanzania: A Review
Wajihu Ahmada1, Jamidu H. Y. Katima1, Richard Kimwaga2, Rob Van Deun3
1Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania
2Department of Water Resources Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania
3Department of Agro- and Biotechnology, Thomas More University College, Belgium
Correspondence to: Wajihu Ahmada, Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Municipal and domestic wastewater contains various pathogenic micro-organisms which, depending on their concentration pose a risk to human health. Therefore their presence must be reduced or eliminated before treated wastewater is reused or released into the environment. Studies conducted by researchers suggest that treatment wetlands are the best systems in removing pathogens from wastewater. Further, the literature reveals that some of the treatment wetland systems achieved up to 2 – 4 log10 removal efficiencies. Effective removal of pathogens in treatment wetland systems calls for an understanding of the processes responsible for pathogen removal. One way of understanding these processes is through the use of mathematical model equation. However, in the case of Horizontal Subsurface Flow (HSSF) constructed wetlands, there is no apparent mathematical model equation explaining pathogens removal processes. On the other hand, treatment wetland systems in Tanzania fail to remove pathogens as intended by designers probably and partly due to improper operation and poor maintenance. This article presents the review of treatment wetland performances in removing pathogens in Tanzania conducted in prior studies. It is also geared toward understanding the removal mechanisms responsible for pathogens removal in these treatment systems.
Keywords: Design equation, Horizontal Subsurface Flow Constructed Wetland, Pathogens, Removal mechanisms, Treatment wetland, Waste stabilization pond
Cite this paper: Wajihu Ahmada, Jamidu H. Y. Katima, Richard Kimwaga, Rob Van Deun, Pathogens Removal from Wastewater Using Sustainable Treatment Wetlands in Tanzania: A Review, American Journal of Environmental Engineering, Vol. 8 No. 2, 2018, pp. 17-24. doi: 10.5923/j.ajee.20180802.01.
Article Outline
1. Introduction
- Treatment wetlands have been established in Tanzania in the past two decades to treat both industrial and municipal wastewater [1]. Constructed Wetlands (CW) researches in Tanzania begin in 1998 at the University of Dar es Salaam, in which the first system was installed to treat wastewater effluents from Waste Stabilization Ponds (WSP) of the University of Dar es Salaam [2], [1]. Over 14 years of successful studies (1998 – 2012), fifteen CWs were established in Tanzania by the “WSP and CW Research group” of the University of Dar es Salaam [3]. In other hand Waste Stabilization Ponds were established in Tanzania in the early 1960’s to deal with problems exacerbated with sewage effluents from industries [4]. WSPs are wastewater treatment options recommended by the government of Tanzania [4]. The effluents from these treatment systems (WSPs and CWs) are used by the locals for irrigation farming, fish farming and sometimes domestic animal feeding due to inaccessibility of fresh water [5], [6], [7]. However reuse of inadequately treated wastewater can cause adverse health risks to humans because of the excreted pathogens it contains [8]. The recommended threshold limit for microbiological effluent quality to protect public health from wastewater exposure, is less than 1 nematode egg/L and less than 1000 cfu/100 mL. [9]. Therefore without appropriate means of removing pathogens from wastewater, more cases of cholera outbreaks and other waterborne diseases are more likely to persist in Tanzania. It is known that controlling frequent outbreak of infectious diseases is more expensive [4].
2. Performance of Wastewater Treatment Systems in Removing Pathogens in Tanzania
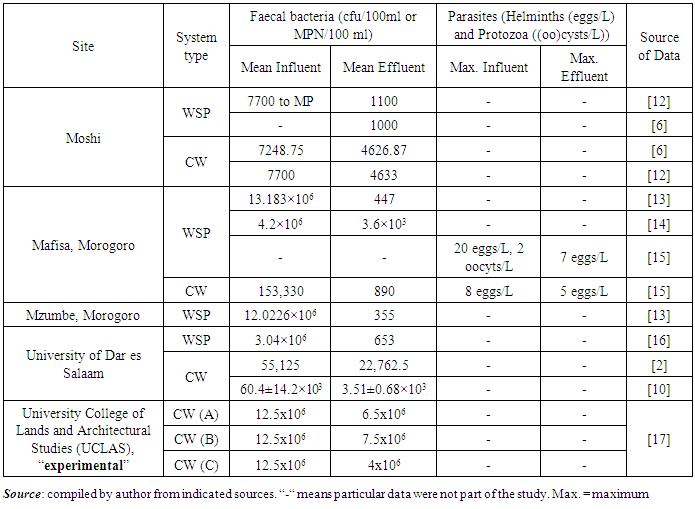
- Constructed Wetlands and Waste Stabilization Ponds have been reported with good performance in treating wastewater worldwide [10]. Although these systems are being good in dealing with wastewater pollutants, some of prior studies carried out in Tanzania show inefficiencies of these systems in eradication of pathogens to the desired levels prescribed by WHO for wastewater reuse. This is elaborated in Table 1.
|
3. Mechanisms for Pathogens Removal
- WSPs and CWs systems remove pollutants from wastewater by mimicking processes found in natural wetlands [18]. In most cases both systems are designed to remove organic matter, Nitrogen and Phosphorus. However pathogens removal from wastewater are also partly considered in the design [8], [18], [19]. In WSPs, systems are configured in such a way that the maturation pond is intentionally designed for removing pathogens [19], [20], [21]. In CW systems, pathogens are mainly removed by filtration, natural die-off, sedimentation, exposure to biocides excreted by wetland plants, and UV irradiation [18], [22]. But in case of horizontal subsurface flow constructed wetland (HSSF) solar intensity is insignificant in removing pathogens [23].Both WSP and CW systems should be improved so that they may efficiently remove the pathogens especially bacteria indicator organisms, helminth eggs, and protozoa cysts. Many studies conducted have concentrated on modelling the systems for the removal of faecal bacteria and helminths in the WSPs. Little information is available in the case of CW for the pathogens removal from design to implementation. Several treatment wetland systems have been constructed and implemented in different countries, but less microbial information is available [24]. To achieve 100% removal of helminth eggs, it is suggested to use Horizontal Subsurface Flow constructed wetland with up to 25 m bed length [8]. Removal of pathogens is influenced by different associated mechanisms in the wetland system.
3.1. Bacteria and Viruses Removal Mechanisms
- Removal mechanisms of bacteria indicator organisms are the most commonly studied in the previous research less information is discussed for specific viruses, bacteria, protozoan cysts and helminths [25], [26]. The mechanisms on the die-off of viruses in WSP are not well understood. Their removal are considered being enhanced mainly by sedimentation followed by adsorption onto solids that results from algae die-off and other bio solids [27]. Oragui et al. [28] investigated faecal coliform and rotavirus in several WSP series in northern Brazil. Each series was comprised of anaerobic, facultative and three maturation ponds, with an overall retention time of 10 – 25 days. Faecal coliforms were reduced from 3×107 cfu/100 ml to ~50 cfu/100 ml, achieving the removal efficiency of 99.9%; number of rotavirus was reduced from 1×104/L of raw wastewater to < 2 viruses/L of effluent, achieving removal efficiency of 99.9%. These results show better performance of WSP in dealing with faecal bacteria due to system associated factors.Several factors that explain the die-off of faecal bacteria in WSP have been proposed, and they are grouped in a light-mediated process or a dark-mediated process [27]. The dark- mediated process includes sedimentation of bacteria adsorbed in to settleable solids, predation by protozoans and die-off due to senescence and starvation. External factors that aid dark- mediated process includes; pond depth (the deeper the pond, the greater darkness in deeper section), organic loading. The light-mediated factors include, temperature and time, high light intensity, high pH, and high dissolved oxygen. These factors are explained in detail in the following sections. Table 2 presents source of data used to create Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 developed by author.
|
3.1.1. Time and Temperature
- Temperature is the result of the solar intensity on surface of the pond, the longer the pond is exposed to light-mediated factors the greater the faecal bacteria inactivation rate [19], [27]. From the data analysis of different WSPs (Figure 1) and CWs (Figure 2) studies carried out in Tanzania show that faecal bacteria concentration were not well correlated with temperature ( r = 0.16 for WSP and 0.66) as compared to that reported by Liu et al. [29] (r = -0.50) this may be due to difference in weather conditions in which Liu et al. [29] study was conducted at high temperature range (-15 – 26oC).
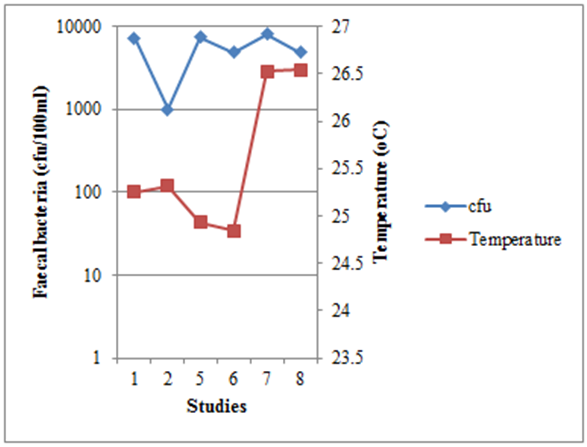 | Figure 1. The relationship between faecal bacteria concentration and temperature in WSPs, from different studies in Tanzania |
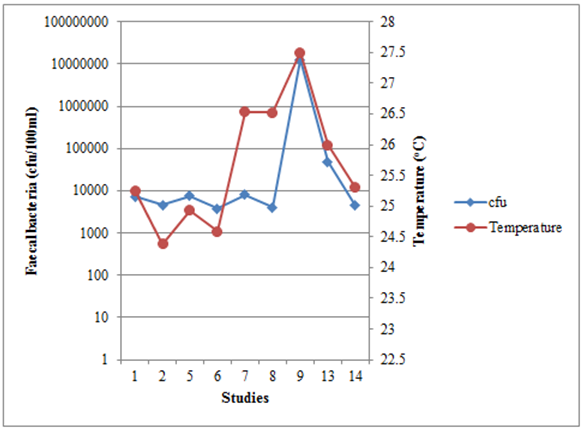 | Figure 2. The relationship between faecal bacteria concentration and temperature in CWs from different studies in Tanzania |
3.1.2. pH
- Wastewater pH ≥ 9.4 increases faecal bacteria die-off very rapidly [27]. High pH is the light mediated factor as it is enhanced by the pond algae. High pH values (above 9) in ponds are influenced by the rapid photosynthesis of pond algae which consumes CO2 faster than it can be produced by heterotrophic bacteria during respiration [19]. The absence of dissolved CO2 in the pond disturbs the equilibrium of CO2 – bicarbonate – carbonate, and consequently bicarbonate and carbonate ions dissociate as shown in equation (1) and equation (2).
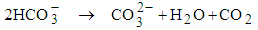 | (1) |
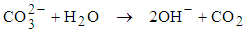 | (2) |
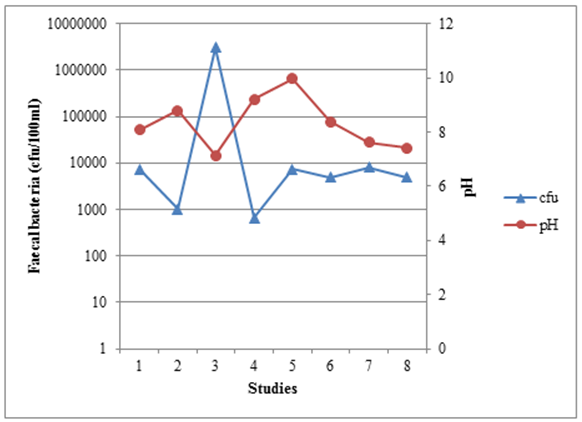 | Figure 3. The relationship between faecal bacteria concentration and pH in WSPs from different studies in Tanzania |
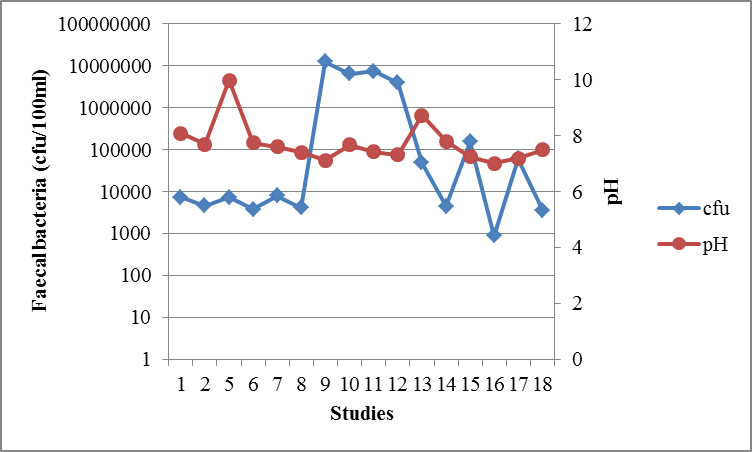 | Figure 4. The relationship between faecal bacteria concentration and pH in CWs from different studies in Tanzania |
3.1.3. Dissolved Oxygen (DO) and Light Intensity
- Light of wavelength up to 700 nm can damage faecal bacteria [30]. However light of wavelength below 450 nm was not an important factor in WSP since it is completely absorbed in the first few mm in the pond downward [27]. The UV wavelengths that can be absorbed by microorganisms, and can effectively disinfect them, comprise the range of 240 – 280 nm [22]. However light of wavelength more than 450 nm can only damage faecal bacteria in the presence of both dissolved oxygen and dissolved sensitizer such as the humic substances. Both dissolved oxygen and humic substances are required for light induced damage of faecal bacteria (referred as photo-oxidation damage) [27], [29]. Photo-oxidation is the process whereby exogenous or endogenous sensitizer absorb light energy and transfer it to other molecules leading to the formation of reactive oxygen species (ROS). ROS react with pathogens and adversely cause cells damage [29]. Therefore an increase in DO concentration is considered to raise the effect of photo-oxidation. Dissolved Oxygen increase in the WSP is associated with algae photosynthesis process.However, photo-oxidation induced disinfection is not only dependent on DO concentrations but also enhanced significantly by sunlight intensity. The light – oxygen – humic substance damage is influenced by intracellular pH values greater than 7.7. Therefore the algae in pond are essential for inactivation of faecal bacteria; since they raise oxygen levels in pond during photosynthesis process and induce high pH values in wastewater. The combination of high light intensity, high dissolved oxygen, high pH and humic substance inactivate bacteria as follows. Humic substance absorbs light and then forms oxygen radicals (e.g. H2O2) after reacting with oxygen by photo oxidation process. Resulting oxygen radicals damage the cell membrane causing the cell to die, and high pH enhances the cell damage in the same way [27]. The analysis of data from prior studies conducted in Tanzania (Figure 5) shows that, the DO increase in WSPs lead to sharp decrease in faecal bacterial concentration and they were well correlated by (r = -0.80). Also in CW (Figure 6) the DO concentrations and faecal bacterial concentrations were well correlated by, r = -0.74.
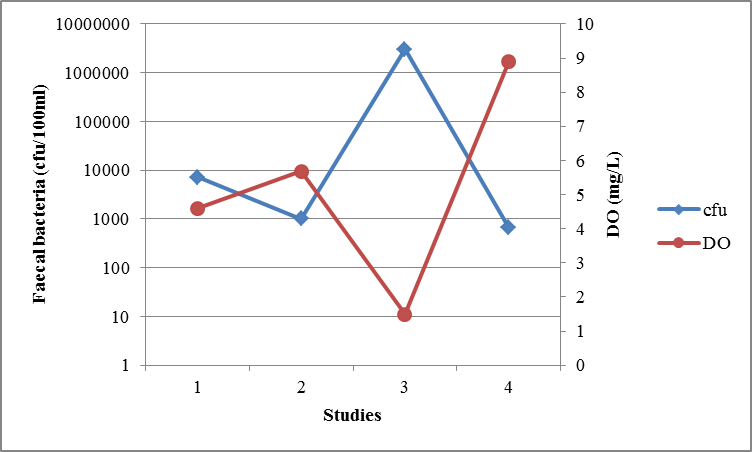 | Figure 5. The relationship between faecal bacteria concentration and DO in WSPs from different studies in Tanzania |
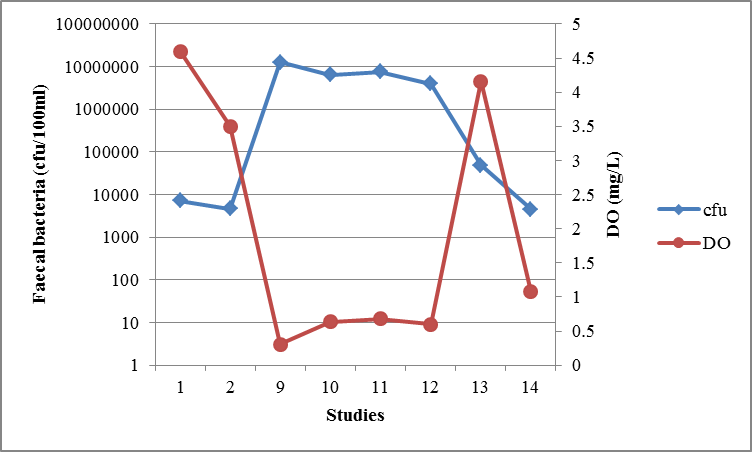 | Figure 6. The relationship between faecal bacteria concentration and DO in CWs from different studies in Tanzania |
3.2. Helminth Eggs and Protozoa Cysts Removal
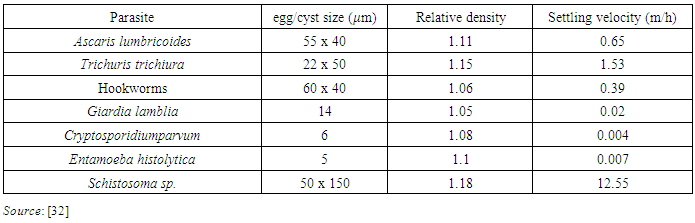
- Studies conducted by Ayres et al. [31] Revealed that helminth eggs and protozoa cysts are mainly removed by sedimentation in WSP. The sedimentation rate is highly influenced by the settling velocity of a specific parasite as indicated in Table 3. To have sufficient removal of helminth eggs and cysts there should be enough hydraulic retention time at a given length/width ratio of WSP system (at least hydraulic retention time of more than 9 days is required to achieve 99% removal) [31].
|
4. Design Equations and Modelling of Pathogens Removal in WSP and CW
- Design equations and models provides a better understanding on the mechanisms and factors that influence pathogens removal in WSP or CW. Majority of models developed concentrated on bacteria indicator organisms as representative to pathogens quality of wastewater effluents. However some studies treated them separately, in which parasites eggs were also considered in separate equation [31].
4.1. Design Equation and Modelling Faecal Bacteria Removal
- Faecal coliform is the most commonly used as indicator organism group to define the presence of pathogens in wastewater [22]. Their removal in Free Water Surface wetlands (FWS) and WSP was considered to follow the first order removal kinetics [19], [22], [27]. Equation (3) below, describes the first order removal kinetics of E. coli [27].
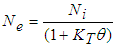 | (3) |
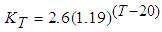 | (4) |
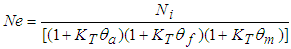 | (5) |
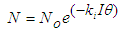 | (6) |
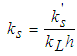 | (7) |
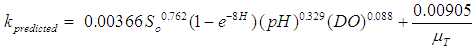 | (8) |
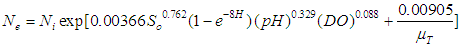 | (9) |
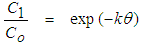 | (10) |
4.2. Design Equation and Modelling of Parasites Eggs Removal
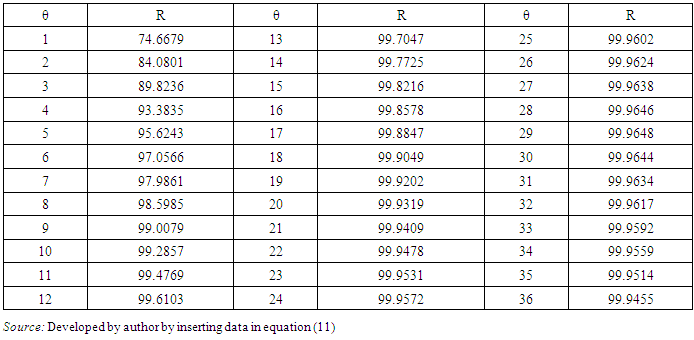
- Ayres et al. [31] developed the design equation for human intestinal nematode eggs removal in Waste Stabilisation Ponds. Equation (11) is the design equation developed, with lower confidence limit of 95% for the design of ponds to meet the WHO microbiological guidelines.
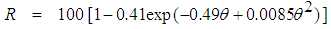 | (11) |
|
5. Conclusions
- From reviewed literature on the removal of pathogens from wastewater using sustainable treatment wetlands, it can be concluded that: The study on mechanisms responsible for removing pathogens is still lacking, hence needs more investigations to speculate what might be promoting or hindering the removal process especially in Horizontal Subsurface Flow constructed wetlands.In case of Horizontal Subsurface Flow, there is no apparent kinetic model for better understanding the removal processes. Therefore the model governing pathogens removal process in HSSF is required and should be developed by considering all factors with significant influence.Finally this can be achieved through formal research by studying, the performance of the wetland systems, factors promoting pathogens inactivation and detention, configuration of the available systems, and manipulation of operational parameters in pilot scale model with improved configuration.
ACKNOWLEDGEMENTS
- The authors would like to express sincere thanks to Mr. Abdallah Zacharia (from Department of Parasitology – Muhimbili University of Health and Allied Science) for contributing his knowledge based on parasitology. Also special thanks to the department of Chemical and Mining Engineering and Department Water Resources Engineering of the University of Dar es Salaam for easing accessibility of materials and better environment for preparation of this article.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML