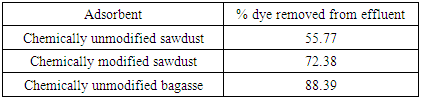Md. Azharul Hossain 1, 2, Shohel Ahmed 1, 2, Jamal Haider Hasib 2, 3, Santanu Das 1, 2, Ashique Ul Haque 1, 2, Rajon Ahmed 1
1School of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan, China
2Department of Textile Engineering, Southeast University, Bangladesh
3School of Textile Science and Engineering, Wuhan Textile University, Wuhan, China
Correspondence to: Md. Azharul Hossain , School of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan, China.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Adsorption is typically used in wastewater treatment to remove toxic or recalcitrant organic pollutant (specially Halogenated but also non halogenated) and to a lesser extent, inorganic contaminants, from the waste water. It finds applications in tertiary wastewater treatment as a polishing step before final discharge. In this study three types of adsorbent were used for removal of color from textile dyeing effluent.
Keywords:
Absorbent, Sugarcane bagasse, Saw dust, Chemical Modification
Cite this paper: Md. Azharul Hossain , Shohel Ahmed , Jamal Haider Hasib , Santanu Das , Ashique Ul Haque , Rajon Ahmed , Remedies of Textile Dyeing Effluent by Adsorbents Produced from Indigenous Agricultural Waste Products, American Journal of Environmental Engineering, Vol. 7 No. 5, 2017, pp. 107-111. doi: 10.5923/j.ajee.20170705.01.
1. Introduction
Textile industry is considered as one of the most pollution oriented industry in world. Among those Dyeing, printing & finishing industry imparts foremost portion of pollution especially aquatic pollution to the environment. Among all of the industries are around dyeing and finishing industries. But the very disappointing feature is counteracted by the pollution it is causing through continual discharging of effluent mostly colored i.e. dye effluent.Dyes are a type of organic compounds that can provide bright and lasting color to other substance. There are more than 100,000 dyes available commercially [1], which are specifically designed to resist fading upon exposure to sweat, light, water, and oxidizing agents and, as such, are very stable and difficult to degrade. [2] In this study, the feasibility of chemically untreated sugarcane bagasse and HCl treated and untreated sawdust has been investigated as adsorbent for the removal of dyes from the effluent of a particular industry named ONETEX PVT LTD, in Bangladesh where reactive dyes are usually used. Batch adsorption studies were performed under various parameters such as pH, agitation time, temperature and adsorbent doses. [3]
2. Material Preparation
2.1. Preparation of Adsorbent from Sugarcane Bagasse
Sugarcane bagasse was collected from local sugarcane crusher and then the collected bagasse was washed several times to remove dust. It was then dried in sunlight and the hard outer part i.e. the husk of sugarcane was removed. The dried portion was then pulverized into fine grains in a local mill and finally the fine grained bagasse adsorbent was produced. Prepared adsorbent of bagasse was stored in a dry and moisture free place, and it was termed as unmodified bagasse. [4]
2.2. Preparation of Adsorbent from Saw Dust with Chemical Modification
Sawdust particularly hardwood sawdust was collected from a local saw mill and dried in air for one day and larger particles were removed with a sieve of size 1mm x 1mm. 10g of sawdust was suspended in 400ml water and 10ml of HCl was added to the suspension. The mixer was stirred for one hour at 85°C ± 2°C in a hot plate magnetic stirrer. The sawdust was then filtered and dried in oven at 50°C and termed as acid activated saw dust. [5]
2.3. Preparation of Adsorbent from Saw Dust without Chemical Modification
Hardwood sawdust was collected from a local saw mill and dried in air for one day. The larger particles were removed with a sieve of size 1mm x 1mm. Sieved sawdust was then stored and termed as unmodified sawdust. [6]
3. Experimental Part
The color of the effluent collected from ONETEX PVT LTD. was too much concentrated that its absorbance was out of UV- VIS spectrophotometer’s limit, even its 10 times dilution was not sufficient to get a sharp absorbance spectra. So, it had to be diluted for more than 10 times. Dilution was done by adding distilled water. For convenience in calculation, the original dye effluent was diluted to 20 times of its original volume to make the sample effluent solution.In each experiment, 100ml sample of colored effluent was taken in a beaker and heated on a hot plate until the desired temperature level was reached. Then different weighed amount of dry activated adsorbent was added to the effluent sample on a hot plate magnetic stirrer. It has been investigated to optimize different parameter such as dosing, agitation time, temperature and pH. In this study, 0.3g, 0.4g, 0.5g and 0.6g of sorbent was investigated in order to find out the appropriate dosing of adsorbent with desired degree of color removal. It was stirred well and kept for different period of time at different temperature; and the liquor was sampled at time of intervals of 10, 20, 30, 40 and 50 minutes. It was studied to find out the effect of heat, conducting the experiment at different temperature of 30°C, 40°C, 50°C, 60°C and 70°C. The most important parameter, the effect of pH value was also investigated. The experiment was carried out at different pH value from 1-5. The sample effluent after treatment was filtered using what man 40 filter paper. The obtained filtrate was allowed to cool to room temperature and analyzed in the spectrophotometer. All the absorbance measurements were carried out in a UV-VIS spectrophotometer (UV 1700 series).
4. Result and Discussion
4.1. Sorption Study
A comparative study of sorption of dyeing effluent which is actually reactive dye effluent was carried out by using chemically modified and unmodified sawdust and chemically unmodified sugarcane bagasse as sorbents. The results of dye removal are shown in Table 1. The experiment was performed by agitating 0.5g of each sorbent in 100ml of dye effluent solution at 30°C and pH-2.0 for 20 minutes. The chemically untreated sugarcane bagasse showed the best adsorption behavior, where dye removal was 89% while upon further reducing to pH-1.0 the adsorption was found 92%.Table 1. Comparative study of dye adsorption by sawdust and sugarcane bagasse at pH-2.0, 30°C
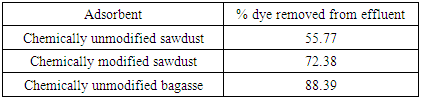 |
| |
|
4.2. Effect of pH
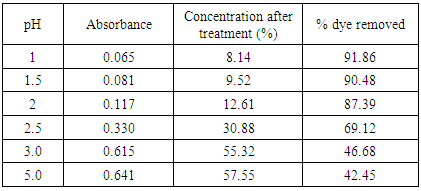
The pH value of dye solution (reactive dye) is an important influencing factor in the dye removal study. It was found that increasing pH has negative effect on dye removal from textile dyeing effluent. [7] In order to find out the optimum pH value for the removal of color from the dye effluent were adjusted to pH 1 to 5. It was verified that the sugar cane bagasse is mainly consisting of natural cellulosic fibers and these fibers are negatively charged due to the presence of hydroxyl groups of cellulose [8]. Depending on pH, these groups may change their charges. At low pH condition, most of the potential binding sites on sugarcane bagasse are protonated and the surface of sorbents is surrounded by hydronium ions. [9] That’s why the acidic pH system showed good adsorption behavior for the reactive dye effluent solution. Solution pH affects both aqueous chemistry and surface binding sites of the adsorbents.Results (absorbance at 559.5nm) of the effect of pH are presented in table 2, 3 & 4 for the treatment with bagasse and sawdust respectively at different temperature.Results for Bagasse Treatment:Dose: 0.5gDuration: 20 minutesTable 2. Effect of pH in treatment of effluent by bagasse at 30°C
 |
| |
|
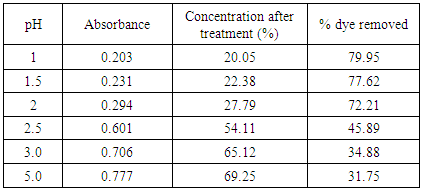
Table 3. Effect of pH in treatment of effluent by bagasse at 60°C
 |
| |
|
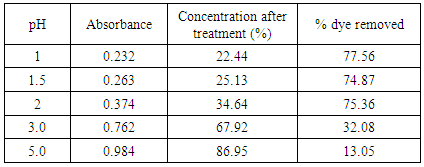
Results for Sawdust Treatment:Dose: 0.5 g, Duration: 20 minutes Table 4. Effect of pH in treatment of effluent by HCl activated sawdust at 60°C
 |
| |
|
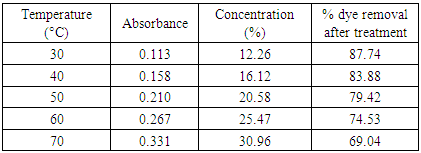
The removal of reactive dye treated with unmodified bagasse increased from 43.45 to 92.0% and from 30.75% to 79.95% with the decrease of pH from 5 to 1 at 30°C and 60°C respectively while in case of HCl activated sawdust treatment the % dye removed increased from 13.05% to 77.56% at 60°C. Reactive dyes are known to ionize to a high degree in aqueous solutions to form colored anions due to the sulfonate groups in their structures. Two sulfonate (–SO3−) groups of reactive dye are easily dissociated and have negative charges in the aquatic environment. As the pH of the system decreases, the protonated surface groups (Si–O–N+H2–C) facilitate the sorption of negatively charged dye. The number of positively charged sites increases resulting in an increase of binding sites for anionic dye (reactive dye) molecules [10]. The deprotonation of surface groups in high pH range results in the electrostatic repulsion between the anionic dye and negatively charged sites. This has contributed to the decreased uptake of reactive dye [11] in alkaline condition. Therefore, it is suggested that the optimum pH for the removal of color from textile dyeing effluent is between pH 1-2.0. [12]Results of HCl activated sawdust treatment: Results (absorbance at 559.5nm) are presented in table 6-16 and plotted in figure 5-7 for HCl activated sawdust at different temperature & pH.Dose: 0.5gTemperature: 60°CDuration: 20minutesTable 5. Treatment of effluent by HCl treated saw dust at pH 5
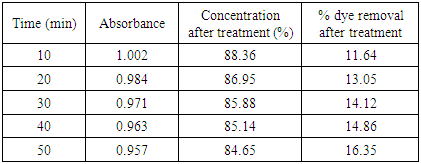 |
| |
|
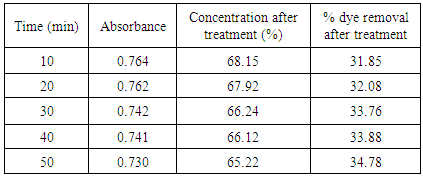
Table 6. Treatment with activated saw dust at pH 3.0
 |
| |
|
Table 7. Treatment with activated saw dust at pH 2.0
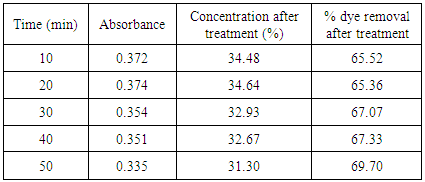 |
| |
|
Table 8. Treatment with activated saw dust at pH 1.5
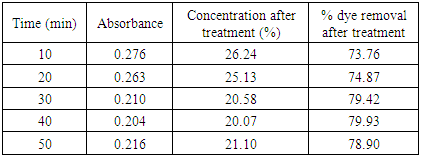 |
| |
|
Table 9. Treatment with activated saw dust at pH 1.0
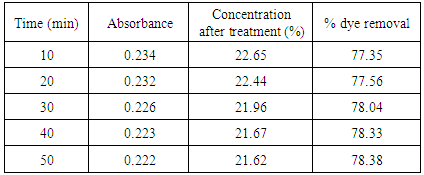 |
| |
|
Table 10. Treatment with bagasse at pH 3.0
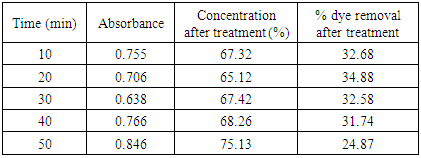 |
| |
|
Table 11. Treatment with bagasse at pH 2.5
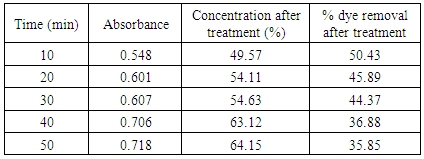 |
| |
|
Table 12. Treatment with bagasse at pH 2
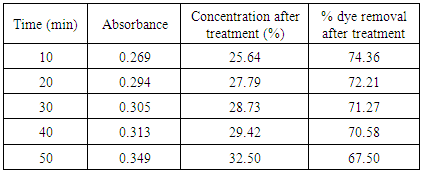 |
| |
|
Table 13. Treatment with bagasse at pH 1.5
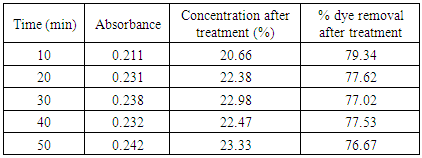 |
| |
|
Table 14. Treatment with bagasse at pH 1.0
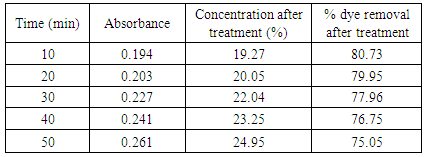 |
| |
|
Table 15. Effect of time in treatment of effluent by bagasse at pH 1.5
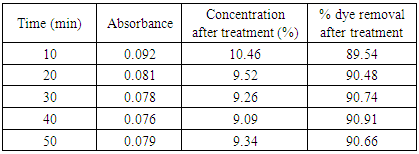 |
| |
|
Dosing optimization at pH-2.0 (30°C & 60°C)Table 16. Effect of dosing in treatment of effluent by bagasse at pH 2, 30°C for 20 minutes
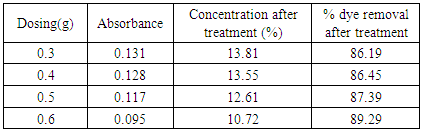 |
| |
|
Table 17. Effect of dosing in treatment of effluent by bagasse at pH-2, 60°C for 20 minutes
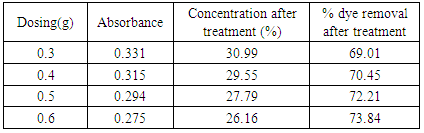 |
| |
|
Dosing optimization at pH-1.0, 60°CTable 18. Effect of dosing in bagasse treatment at pH 1.0, 60 degree Celsius
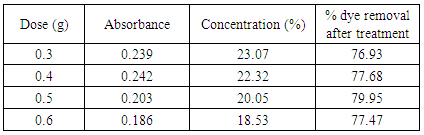 |
| |
|
Table 19. Temperature optimization with bagasse (0.5g) at pH 2.0
 |
| |
|
5. Conclusions
Batch adsorption study for the removal of dye from the dyeing effluent of ONETEX PVT LTD. using activated adsorbents of sugarcane bagasse and saw dust has been investigated for different variables viz pH, agitation time, adsorbent doses and temperature. This study used the sugarcane bagasse as adsorbent without chemical modification and sawdust with and without chemical modification.The following conclusion has been drawn from the above investigation:1. The efficiency of color removal from the dye effluent was found to be best in case treatment with chemically unmodified sugarcane bagasse. This investigation showed that at a certain condition the efficiency of color removal by chemically unmodified bagasse was 88.39% while the efficiency by chemically modified and unmodified sawdust was 72.38% and 47.77% respectively.2. Effect of different variables was investigated to find out the optimum value for each variable in the treatment of textile dyeing effluent. Effect of pH was found to be the most significant factor in this study. The original pH of the effluent was 10.2 and at this pH, almost no dye could be removed using any means of adsorbent. Decreasing pH was found to be effective. The more pH was decreased, the more dye was removed. The best result in this study was found at pH-1.0 where 91.86% of color removal was achieved. However, at low pH this effect was little bit insignificant cause it was observed that the efficiency of color removal at pH-1.5 was 90.48%, at pH-2.0 was 87.39% and at pH-2.5, it was 69.12%. So it can be inferred from the date given here that pH 1.0 to pH-2.0 can be considered to be optimum.3. Effect of agitation time was investigated in this batch adsorption study at different pH and temperature. It was observed that agitation time has no substantial effect on the treatment of textile dyeing effluent. The contact time period of 10 to 20 minutes was found sufficient for maximum color removal. Any time increase above 20 minutes has very negligible effect.4. Adsorbent dosing had positive effect in treatment of textile dyeing effluent. [13] Any increase of adsorbent doses, increased the % dye removal. 0.3g, 0.4g, 0.5g and 0.6g of adsorbent doses were investigated to get the optimum value. It was found that at low pH, this effect was not very significant. So 0.3 to 0.5g of adsorbent can be considered optimum to get the desired amount of color removal.5. The second most important factor in the treatment of dyeing effluent found in our investigation was temperature. Similar to pH, decreasing temperature was found to increase % dye removal significantly. So, adsorption of dye from textile effluent was exothermic process. The room temperature (30°C) was most effective than any other above temperature. 6. It was observed that the dyeing effluent from ONETEX PVT LTD. was highly concentrated. It was almost impossible to remove any significant amount of coloring compounds by activated adsorbents while using original effluent sample. Moreover, the concentration to the dye effluent was so intense that it was out of detecting limit of UV-VIS spectrophotometer. That’s why it was very obvious to dilute that sample before treatment. Effluent was diluted 20 times of its original amount by distilled water, and then it was much easier to treat the effluent by activated adsorbent and to get the absorbance in UV-VIS spectrophotometer was 92% of the dye effluent could be removed. 7. It was found that the COD of the effluent is highly reduced after treatment with activated adsorbent especially chemically unmodified sugarcane bagasse. Before treatment COD was 453 while after treatment it was only 10.5 which made our investigation very warranted.It can be inferred from the above discussion that un treated sugarcane bagasse can be used efficiently in treatment of textile dyeing effluent of reactive dye. However, further investigation is required to justify the feasibility of sugarcane bagasse in treatment of effluent of other type of dyes.
References
| [1] | Boyter HA (2007). Environmental legislations USA. Environmental aspects of textile dyeing. Woodhead, Cambridge, England. |
| [2] | S. M. Metev and V. P. Veiko, Laser Assisted Microtechnology, 2nd ed., R. M. Osgood, Jr., Ed. Berlin, Germany: Springer-Verlag, 1998. |
| [3] | D. S. Kharat, Preparing Agricultural Residue Based Adsorbents for Removal of Dyes From Effluents - A Review. |
| [4] | Karnitz, Osvaldo, et al. "Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane gasse." Bioresource technology 98.6 (2007): 1291-1297. |
| [5] | Khattri, S. D., and M. K. Singh. "Removal of malachite green from dye wastewater using neem sawdust by adsorption." Journal of Hazardous Materials 167.1 (2009): 1089-1094. |
| [6] | Tramontina J, Marchado G, Azambuja D S, Piatnicki C M S and Samios D, Material Research, 2001,4, 195. |
| [7] | Ramakrishna, Konduru R., and T. Viraraghavan. "Dye removal using low cost adsorbents." Water Science and Technology 36.2-3 (1997): 189-196. |
| [8] | Jústiz-Smith, N.G.; Virgo, G.J. and Buchanan, V.E. (2008). ‘Potential of Jamaican banana, coconut coir and bagasse fibers as composite materials.’ Mater Charact. 59: 1273-1278. |
| [9] | Wong, S. Y.; Tan, Y. P.; Abdullah, A. H. & Ong, S. T. (2009): The Malaysian Journal of Analytical Sciences, Vol 13 No 2 185 – 193. |
| [10] | Chiou, M.S. and Li, H.Y. (2003). ‘Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads.’ Chemosphere. 50: 1095-1105. |
| [11] | Tor, A. and Cengeloglu, Y. (2006). ‘Removal of congored from aqueous solution by adsorption onto acid activated red mud’. J. Hazardous Mater. B138: 409-415. |
| [12] | Vilar VJP, Pinho LX, Pintor AMA, Boaventura RAR. Treatment of textile wastewaters by solar-driven advanced oxidation processes. J Sol Energy. 2011; 11(9): 1927–1934. doi: 10.1016/j.solener.2011.04.033. |
| [13] | Rao, VV Basava, and S. Ram Mohan Rao. "Adsorption studies on treatment of textile dyeing industrial effluent by flyash." Chemical Engineering Journal 116.1 (2006): 77-84. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML