-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2017; 7(4): 73-81
doi:10.5923/j.ajee.20170704.01

Modeling and Optimization of Structural Steel Corrosion Inhibition using Barely Grass Extract as Green Inhibitor
Ammar Ali1, Sarah Falih1, Nedal Yousif2, Reben Rezgar3, Ibtisam Kamal4
1Dept. of Civil Engineering, Faculty of Engineering, Iraq University College, Basrah, Iraq
2Technical College, Southern Technical University, Basra, Iraq
3Dept. of Chemical Engineering, Faculty of Engineering, Soran University, Kurdistan Region, Iraq
4Soran University, Kurdistan Region, Iraq
Correspondence to: Ibtisam Kamal, Soran University, Kurdistan Region, Iraq.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The work deals with studying the corrosion inhibition of structural steel in acidic medium (0.1M HCl) using barley grass extract as inhibitor. The effect of the concentration of inhibitor (432-768) ppm, time (15.3-176.7) h, and temperature (24-44)°C on corrosion rate, inhibition efficiency and degree of surface coverage of the metal alloy was investigated using Response Surface Methodology (RSM). Fitting of degree of surface coverage with Freundlich adsorption isotherm was conducted. The kinetics parameters of corrosion and inhibition processes including the activation energy and frequency factor were estimated as well as the thermodynamic parameters including the standard free energy of adsorption Δ G°ads, the entropy of activation (ΔS*) and enthalpy of activation (ΔH*). The corrosion rate was found to increase with increasing time and temperature, while the inhibition efficiency was found to increase with increasing the inhibitor concentration and decrease with time and temperature. The RSM analysis results revealed that maximum corrosion rate of about 2.11 mg/h resulted at 42°C and 164 h without using inhibitor compared to 0.26 mg/h at 43°C and 72 h with using the green inhibitor respectively. The value and the negative sign of ΔG°ads indicated that the inhibitor molecules adsorbed spontaneously onto the metal surface via physiosorption. The positive shift of enthalpy of activation in presence of inhibitor reflected that the process of adsorption of the inhibitor on the alloy surface is an endothermic process and the negative values of entropy of activation represented association rather than dissociation of the inhibitor indicating the decrease of system disorder due the adsorption process. According to the estimated results, barely grass extract could be recommended as low cost green inhibitor for acid corrosion inhibition of structural steel used in manufacturing industrial equipment and vessels.
Keywords: Barley grass extract, Inhibitor, Corrosion, Optimization, Modeling
Cite this paper: Ammar Ali, Sarah Falih, Nedal Yousif, Reben Rezgar, Ibtisam Kamal, Modeling and Optimization of Structural Steel Corrosion Inhibition using Barely Grass Extract as Green Inhibitor, American Journal of Environmental Engineering, Vol. 7 No. 4, 2017, pp. 73-81. doi: 10.5923/j.ajee.20170704.01.
Article Outline
1. Introduction
- Steel is an important engineering material because of its outstanding structural and mechanical properties that recommended its use in various fields including oil and chemical industries. However, there are some challenges for applications of the alloy in a large industrial scale. The most important one is its moderate corrosion resistance. When steel structure exposed to an aggressive environment such as oil-well stimulation and other industrial processes including acid pickling, this may result in decrease the mechanical stability and the attractive appearance of the structure, therefore, the steel structure needs to be protected with a high performance treatment and may need to be designed with maintenance in mind if extended life is required. The corrosion of structural steel is an electrochemical process that requires the simultaneous presence of moisture and oxygen. Essentially, the iron in the steel is oxidized to produce rust, which occupies approximately 6 times the volume of the original material consumed in the process. The rate at which the alloy corrosion process progresses depends on the environment surrounding the structure, the most significant factors affected the process are time of wetness and the atmospheric pollution level.To protect steel alloys from being corroded, several techniques are used including conversion surface treatments such as chromating, phosphating, etc., anodizing, electroplating and adding corrosion inhibitors. The key to success protection lies in identifying the corrosivity of the environment to which the structure will be exposed as well as defining the appropriate protection technique. A great number of scientific studies have been devoted to the corrosion techniques (Telegdi et al., 2000; Eliyan et al., 2013), however, adding corrosion inhibitors has been little involved. Corrosion Inhibitors are chemicals that react with a metallic surface, or the environment the surface is exposed to resulted in giving the surface a certain level of protection. For the metals widely employed in the industry such as iron, copper, zinc and aluminum, adding corrosion inhibitors is an effective and convenient method to decrease the corrosion rate (Roberge, 1999). Inhibitors slow corrosion processes by either increasing the anodic or cathodic polarization behavior (Hughes et al., 2016), reducing the movement or diffusion of ions to the metallic surface, or increasing the electrical resistance of the metallic surface (Odewunmi et al., 2015).The addition of corrosion inhibitors is a standard practice in chemical, petrochemical and oil industries to control the internal corrosion of steel structures. Inorganic inhibitor including chromate (Cr2O4-), nitrate (NO2-), molybdate (MoO3-), phosphate (H2PO3-) and silicates have been the most widely used. Organic inhibitors belonging to different chemical families were tested and industrially applied as corrosion inhibitors for steel. These include hydroxyethyl, aminoethyl, and amidoethyl imidazolines (Villamizar et al., 2007), N,N-di (poly oxy ethylene) (Migahed, 2005), and nicotinamide derivatives (Chakravarthy and Mohana, 2008). The inhibition of metal corrosion by organic compounds has been confirmed to be a result of adsorption of organic molecules at the metal surface due to the electrostatic attraction between the electrical charge of the metal surface and the ionic charge or dipole of the inhibitor molecules. Adsorption allows the collection of a thin film that does not react with the metal nor corrosive species, but rather acts as barrier that prevents corrosion of the base material. This phenomenon is influenced by the nature of the metal, the metal surface condition, mode of adsorption, type of aggressive electrolyte and by the chemical structure of inhibitors (Abd El-Lateef et al., 2012; Abbasov et al., 2013). Although much inorganic, organic and macromolecular compounds have been showed good performances as corrosion inhibitors for different metals and alloys, many of these compounds are toxic and do not satisfy completely the requirements obeyed by the environmental protection standards. The new generation of environmental regulations requires the replacement of toxic chemicals with the so-called "Green chemicals". Therefore, the development of non-toxic and environmental friendly inhibitors is considered more important and attractive.On another hand, plant extracts and products are organic in nature, and some of the constituents including tannins, amino acids, alkaloids, and pigments are known to exhibit inhibiting action (Al-Turkustani et al., 2012; Buchweishaija and Mhinzi, 2008; Gadow et al., 2017; Raja and Sethuraman, 2008; Zarrok et al., 2013). Different extracts from agro sources were produced by simple procedures, they have been investigated and showed anticorrosive activities (Abdel-Gaber et al., 2006; Benali et al., 2013; De Gisi et al., 2016 Ngobiri et al., 2015; Pehlivan et al., 2012; Singh et al., 2013; Umoren et al., 2015).To the best of our knowledge, the present work is the first one that describes the using of barely grass extract as corrosion inhibitor. Barley grass is an agricultural by-product; it is the leaf portion of the barley plant that remains after the seeds have been removed from agricultural, household and industrial sectors treatment. Barley grass has been recognized for its high concentrations of amino acids and other constituents. Because of the nontoxicity and eas to produce barely grass extract in high purity. The present work aims to use the discarded barley grass as eco-friendly source to produce green corrosion inhibitor for structural steel in acidic medium. Using this agriculture waste will provide two advantage to environment. The volume of agro waste could be reduced and the low-cost inhibitor could be easily produced for reducing the pollution problems arising from structural steel corrosion.
2. Experimental
2.1. Methodology
- Response Surface Methodology (RSM) is a statistical tool used to investigate the interaction between several illustrative variables and one or more response variables. The process of RSM includes designing of a series of experiments for sufficient and reliable measurement of the response and developing a mathematical model of the second order response surface with the best fittings. The Software portable statgraphics plus for Windows software (5.1 version) was used to analyze the data. The mathematical empirical model is defined as:
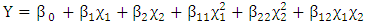 | (1) |
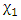 and
and 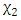 are the independent variables;
are the independent variables; 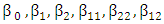 are the regression coefficients. The analyses of variance (ANOVA) are used to determine significant differences between the effects of independent variables (p<0.05). Pareto chart is used to identify the impact level of the independent variables on each considered response. The vertical line (significant front) in Pareto chart determines the effects that are statistically significant at 95% as confidence level. Main Trends and Surface Response, as well as the empirical regression model can be used to optimize the dependent parameter (responses). Theory and applications of RSM are highlighted in the literature (Raymond et al., 2016).
are the regression coefficients. The analyses of variance (ANOVA) are used to determine significant differences between the effects of independent variables (p<0.05). Pareto chart is used to identify the impact level of the independent variables on each considered response. The vertical line (significant front) in Pareto chart determines the effects that are statistically significant at 95% as confidence level. Main Trends and Surface Response, as well as the empirical regression model can be used to optimize the dependent parameter (responses). Theory and applications of RSM are highlighted in the literature (Raymond et al., 2016).2.2. Experimental Design
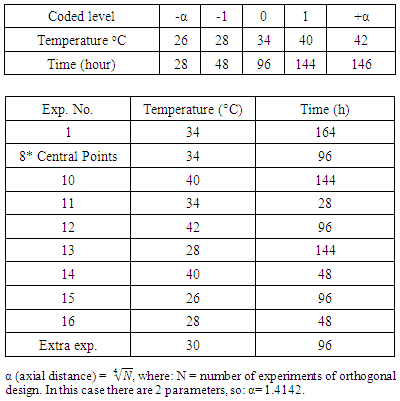
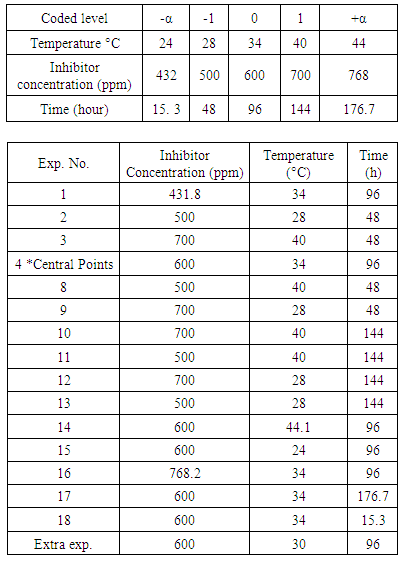
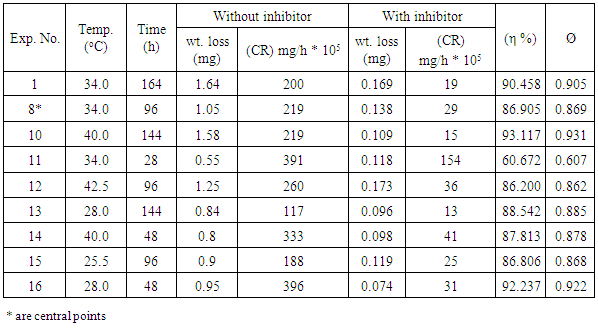
- A central composite design of 16 experiments include 2-operating parameter (with 2*2=4 factorial points, 2*2 star-points and 8 repetitions of central point) was adopted for the experiments used for studying the effect of temperature and immersion time on corrosion parameters of structural steel without using inhibitor, while another central composite design of 18 experiment include 3-operating parameter was established for the experiments used for studying the effect of temperature, time, and inhibitor concentration on corrosion parameters. The analysis for each sample was replicated three times. The experimental design for the experiments without inhibitor and with inhibitor are shown in Table 1 and Table 2 respectively.
|
|
2.3. Preparation of Barley Grass- acid Solutions
- About 100 g of the grass powder was refluxed with 0.1M HCl for about 5 h and was kept overnight to completely extract the basic components. The solution was filtered off and the filtrate was concentrated by rotary evaporator. The resultant gummy material was dried and powdered and weighed accurately by digital micro-balance. The extracted mass was used in preparation of the acid solutions of different concentrations of the inhibitor according to the experimental design.
2.4. Weight Loss Measurements
- The present study employed weight loss as measurement techniques for corrosion to monitor the effect of temperature, time, and concentration of inhibitor on structural steel grade A500A corrosion in 0.1M hydrochloric acid solution in order to determine its viability in process industries. The acid used was of AR grade and used as such. Distilled water was used in the preparation of the test solutions.The structural steel bars were cut to specimens of 2.5 cm × 2.0 cm × 0.6 cm. Each specimen was perforated to give a hole of same diameter, at about 0.5 cm from the length of the specimen. The specimens were abraded with emery papers (grades 320, 500, 800 and 1200) and then washed with distilled water and acetone. They have been weighed accurately. The pre-cleaned and weighed specimens were suspended in beakers containing the test solutions. Tests were conducted under total immersion conditions in (500-mL beakers containing 350 ml of the 0.1M HCl solution in absence and presence of the inhibitor at different temperature and time intervals according to the experimental designs. After immersion time intervals, the specimens were carefully washed in double-distilled water, dried, and re-weighed accurately by analytic digital micro-balance. The weight loss was taken to be the difference between the weight of the specimen at a given time and its initial weight. The corrosion rate (CR) and the inhibition efficiency (η %) were calculated using the following equations:
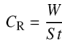 | (2) |
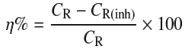 | (3) |
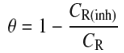 | (4) |
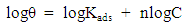 | (5) |
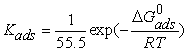 | (6) |
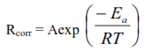 | (7) |
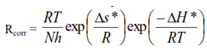 | (8) |
3. Results and Discussion
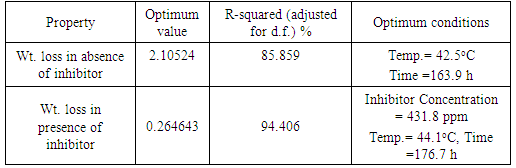
- The RSM analysis results of the impact of temperature and immersion time on structural steel corrosion in 0.1M HCl in absence and presence of the inhibitor are shown in Figure 1 and Figure 2 respectively. A summary of the results is shown in Table 3.
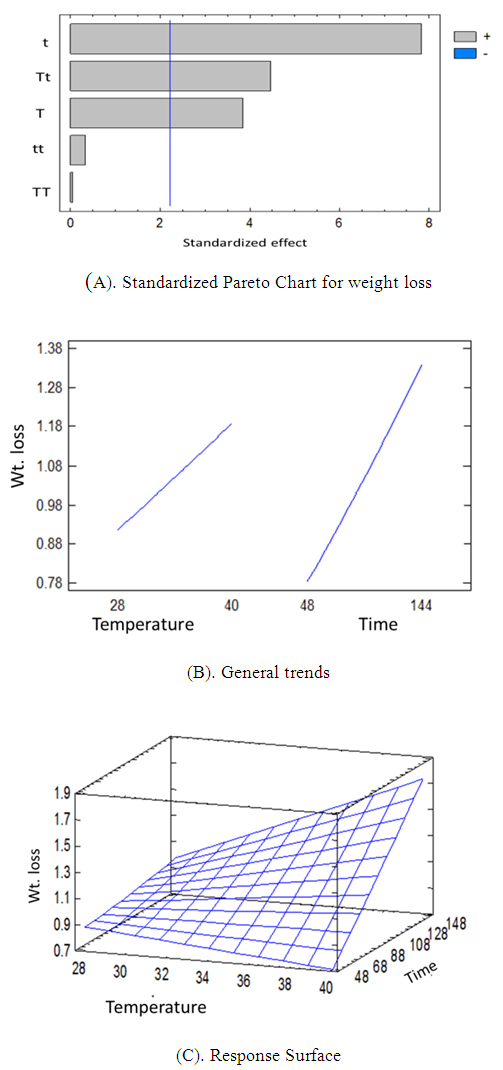 | Figure 1. Pareto chart of the effect temperature and time (A), general trends (B), response surface (C) for corrosion process in absence of inhibitor |
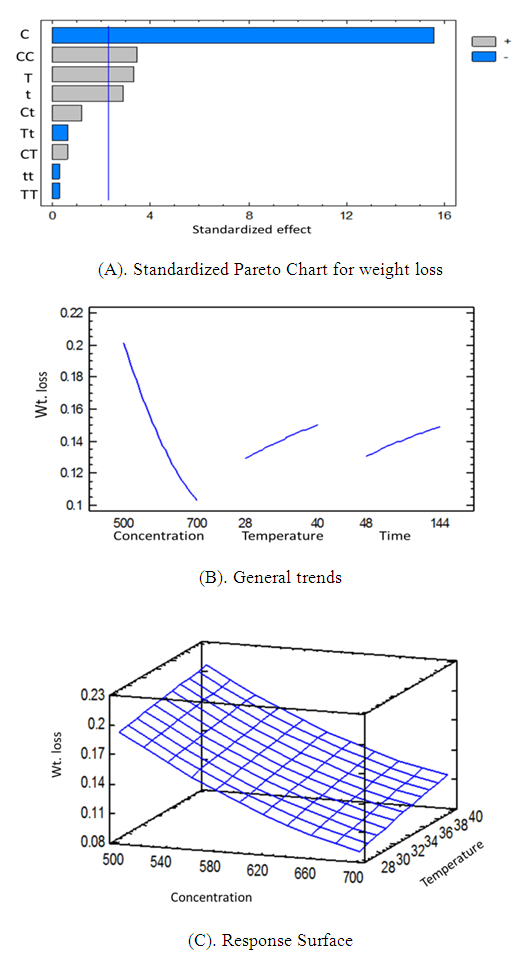 | Figure 2. Pareto chart of the effect temperature, time and inhibitor concentration (A), general trends (B), response surface (C) for corrosion process in presence of inhibitor |
|
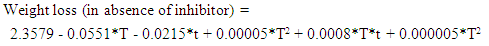 | (9) |
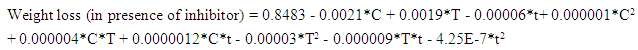 | (10) |
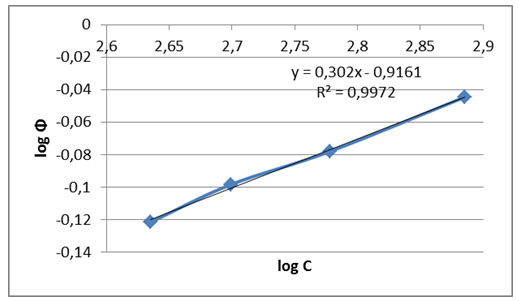 | Figure 3. Freundlich isotherm for corrosion inhibition in presence of different concentrations of inhibitor |
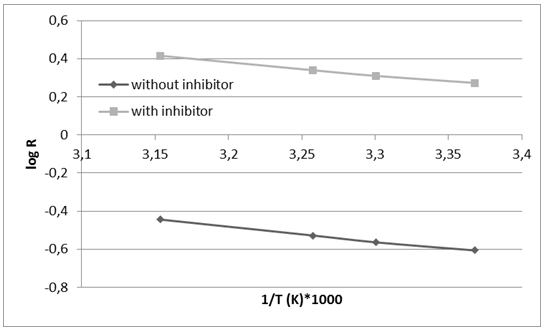 | Figure 4. The Arrhenius plot for the corrosion process in absence and presence of the green inhibitor |
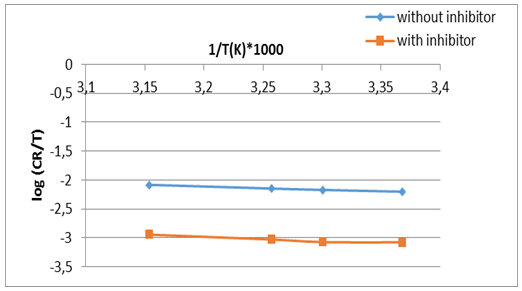 | Figure 5. The Eyring plot for the corrosion process in absence and presence of the green inhibitor |
|
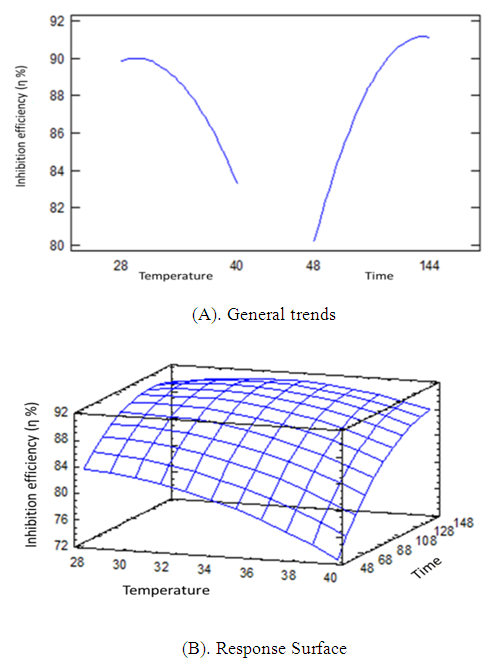 | Figure 6. General trends (A), response surface (B) for corrosion process in absence of inhibitor |
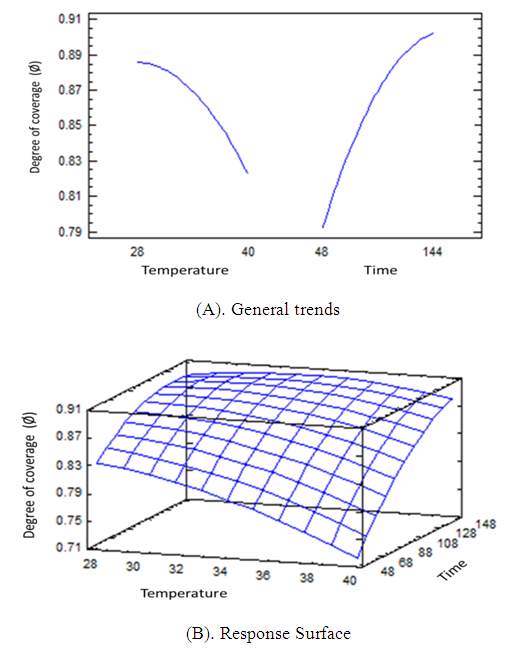 | Figure 7. General trends (A), response surface (B) for the inhibition efficiency (η %) and degree of coverage of the metal surface Ø in the presence of inhibitor (600) ppm |
|
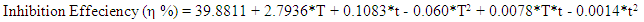 | (11) |
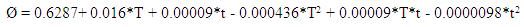 | (12) |
4. Conclusions
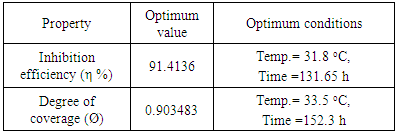
- Barley grass extract has been prepared and tested for inhibition the corrosion of structural steel in acidic medium. The corrosion process was optimized and modeled in absence and presence of the new inhibitor throughout adopted experimental desigs using Response Surface Methodology (RSM). The thermodynamics of the corrosion procecesses was studied. The green extract exhibited a very good performance as inhibitor for structural steel corrosion in 0.1 M HCl such as an optimum reduction in wt. loss of the metal alloy of more than 8 times by adding the inhibitor was predicted. The corrosion process in presence and absence of the green inhibitor seemed endothermic and the inhibition mechanism has been confirmed as physisorption. An optimum values of degree of covarage (0.9034) and Inhibition Efficiency % (91.4136) using 600 ppm of the green inhibitor was obtained which highly recommend using such low cost waste for corrosion inhibition of industrial steel equipment and vesseles.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML