-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2017; 7(3): 58-64
doi:10.5923/j.ajee.20170703.02

Effect of Different Sets of Pleurotus ostreatus and Aspergillus niger Hydrolysis of Cassava Peelings on Bioethanol Yield
Michael N. Worfa1, Moses Mensah2, Benjamin Afotey2, Samson P. Salifu1
1Department of Biochemistry and Biotechnology, College of Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
2Department of Chemical Engineering, College of Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Correspondence to: Benjamin Afotey, Department of Chemical Engineering, College of Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study investigated the effect of three different types of microbial activity on bioethanol yield from cassava peelings. The cassava peelings were pretreated by milling to 2mm particle size, autoclaved and hydrolyzed using Pleurotus ostreatus k910 (white-rot fungi), Aspergillus niger Menae1 (black molds) and a combination of the two fungi. The hydrolysates obtained were subsequently fermented to ethanol using the Saccharomyces cerevisiae (baker’s yeast). The analysis of lignocellulose fractions, fermentable sugars and bioethanol produced were performed using a van Soest, refractometry and gravimetric methods respectively. The effect of the various fungi used for biological pretreatment/hydrolysis on lignocellulose fractions and fermentable sugars released of each substrate were studied. P. otreatus k910 hydrolysis of the cassava peelings yielded an optimum fermentable sugar concentration of 34.11 g/L compared to 28.64 g/l for the A. niger MENAE1. However, the combination of the two fungi for hydrolysis gave the best results for fermentable sugar produced of 36.51 g/l for cassava peelings. The fermentation results revealed that the maximum ethanol yield for cassava peelings is 19.36% (w/w dry biomass).
Keywords: Second-generation bioethanol, Lignocellulose, Autoclave, Refractometer, Fermentation, Pleurotus ostreatus (white-rot fungi), Aspergillus niger (black molds) and Saccharomyces cerevisiae (baker’s yeast)
Cite this paper: Michael N. Worfa, Moses Mensah, Benjamin Afotey, Samson P. Salifu, Effect of Different Sets of Pleurotus ostreatus and Aspergillus niger Hydrolysis of Cassava Peelings on Bioethanol Yield, American Journal of Environmental Engineering, Vol. 7 No. 3, 2017, pp. 58-64. doi: 10.5923/j.ajee.20170703.02.
Article Outline
1. Introduction
- Rising demand for energy as results of rise in global population, urbanization and increasing per capita energy consumption has warranted the search for alternate energy sources. In 2014, 86.3% of the global energy utilization originated from fossil fuels: coal (30%), oil (32.6%) and natural gas (23.7%) [1]. The reliance of the twenty-first-century societies on primary energy sources (fossil fuels) is known to be the reason for escalating climate change challenges and natural resources depletion [2]. The extensive use of petroleum fuels leads to several concerns including; destruction of natural habitats and landscapes and high levels of environmental pollution. In light of these facts, the significance of developing clean, practicable and sustainable energy sources has risen considerably with many scientists working on the possibility of converting biomass into bioethanol to serve as a substitute to fossil fuels [3]. Bioethanol is a product of microbial fermentation of sugars as opposed to synthetic ethanol produced from petrochemical sources. The fermentation of glucose or other sugars in biomass produces ethanol and carbon dioxide expressed as:
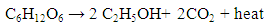 | (1) |
2. Materials and Method
2.1. Sources of Experimental Materials
- The cassava peelings were obtained from cassava dumpsites in Kumasi, Ghana. Spawn of P. ostreatus k910 used for biological pretreatment/enzymatic hydrolysis was obtained from Robart Farms, Kumasi. A. niger MENA1E was also used for biological pretreatment and enzymatic hydrolysis was obtained from Pathology Laboratory of the Faculty of Agriculture, Kwame Nkrumah University of Science and Technology, Kumasi. Baker’s Yeast used for ethanol fermentation was purchased from a grocery store.
2.2. Preparation of A. niger MENAE1Spore Suspension for Hydrolysis
- Under aseptic conditions, 20 ml of the sterile Potato Dextrose Agar (PDA) was poured into sterilized petri dishes and inoculated with pure cultures of A. niger MENAE1. The A. niger MENAE1 was subjected to lactophenol cotton blue staining which confirmed the morphology and spore colouration characteristic of the species. A sterile inoculation pin was used to pick the spores from the pure culture unto the PDA in eight replicates. The inoculated Petri plates were incubated at room temperature for four days after which spores of the mold were collected by flooding the cultures with 20 ml sterile distilled water to dislodge the pores. The spore suspensions were then transferred into a sterile 250 ml Erlenmeyer flask and kept for further use. Serial dilution of 1/10000 ml was prepared. The diluted suspension was mixed thoroughly and a drop was sampled with pipette and the cells population determined using a haemocytometer and light microscope. A. niger isolated from the fungal culture was subjected to lactophenol cotton blue staining which confirmed the morphology and spore colouration characteristic of the species.
2.3. Preparation of Stock Culture of S. cerevisiae for Ethanol Fermentation
- An amount of 2 grams of dry yeast was transferred into 10 ml of nutrient broth, mixed thoroughly and incubated for 24 hours. Aseptically, a loop-full of the 24 hours old culture was then streaked on malt extract agar and incubated at 30°C for 24 hours. Pure culture of a single colony of the yeast was subsequently inoculated into 50 ml of nutrient broth in a sterile 100 ml conical flask and incubated at 30°C for 72 hours in a shaking incubator at 100 rpm and this was used for the ethanol fermentation. Cell population was counted with the aid of a haemocytometer [9].
2.4. Substrate Preparation
- Dried cassava peelings were grinded and sieved with 2 mm size mesh to obtain a uniform size and these served as the substrates. The amount of 50 grams of it was weighed into 250 ml conical flasks containing 50 ml of distilled water and mixed thoroughly. The flasks were then corked with absorbent cotton wool and covered with aluminium foil. These were done in triplicates for eight weeks. The conical flasks with their contents were then autoclaved at a temperature of 121°C, a pressure of 15 psi for 15 min.
2.5. Determination of Moisture Content of Unhydrolyzed Samples
- An amount of 2 grams of the untreated samples was transferred into a cleaned crucible (weight known) in triplicates. The individual crucibles with their contents were put in an oven at a temperature of 110°C for 24 hours, the crucibles were taken out, allowed to cool at room temperature and reweighed. Moisture content was calculated by difference and expressed as a percentage of the initial weight of the samples [10].
2.6. Biological Pretreatment and Enzymatic Hydrolysis of the Substrates with P. ostreatus, k910
- After autoclaving, the substrates were allowed to cool at room temperature. Under aseptic conditions, the bottle containing the spawns of P. ostreatus was shaken to loosen the grains. Ten grams of the grains were weighed into a sterile container and subsequently evenly spread onto the prepared substrates thus a ratio of 1:100 in each of the conical flasks and corked with cotton wool [11]. The experiments were done in triplicates for each substrate. In the experimental control set up, spawns of P. ostreatus k910 were not placed onto the substrate. The inoculated substrates and the controls were incubated at 28°C for a period of 8 weeks with weekly interval terminations of cultures for each substrate for further analysis.
2.7. Biological Pretreatment and Enzymatic Hydrolysis of Cassava Peelings with A. niger MENA1E
- Under aseptic condition, spore suspension (10 ml of 2.2 x 106 cell.ml-1) of A. niger MENA1E was poured unto the substrates in each flask and corked again. In the experimental control set up, spawns of P. ostreatus k910 were not placed onto the substrate. The inoculated substrates and the controls were incubated at 28°C for a period of 8 weeks with weekly interval terminations of cultures for each substrate for further analysis.
2.8. Biological Pretreatment and Enzymatic Hydrolysis of P. ostreatus k910 Hydrolyzed Cassava Peelings with A. niger MENA1E
- The substrates were first hydrolyzed with P. ostreatus k910 and the hydrolysis process terminated weekly and then autoclaved. These served as substrates for the second stage hydrolysis using the A. niger MENAE1 suspension. Under aseptic conditions, 10 ml of the spore suspension was poured onto the substrates in each flask and corked. The experiments were done in triplicates. In the experimental control set up, spawns of P. ostreatus and suspensions of A. niger were not added on the substrate. These were subsequently incubated at 28°C for 5 days.
2.9. Extraction of Fermentable Sugars
- The fermentable sugars were extracted from the hydrolyzed substrates by adding 300 ml of distilled sterile water to the content of the flasks, and fixed on a rotatory shaker at 200 rpm for 30 minutes at room temperature. The mixtures were filtered using filter cloth and the filtrate collected in sterile container. Distilled sterile water of 200 ml was used to thoroughly rinse the conical flask and the rinsed water filtered. The filtrate was centrifuged at 5,000 rpm for 30 min at 28°C and the supernatant stored in a freezer at -20°C until needed for downstream application. The residue was oven-dried at 70°C for 72 hours, milled to homogeneity and stored for chemical analysis.
2.9.1. Determination of Fermentable Sugars
- Fermentable sugars were determined using a digital refractometer (HI96801). Distilled water was used to blank the refractometer and then 3 ml of supernatant was drawn and dropped on a groove of the refractometer. The total sugar values were read, converted from Brix to g/l and recorded.
2.10. Fermentation of Cassava Peelings Hydrolysates to Ethanol
- Ethanol fermentation was conducted using a modified method of Zakpaa et al. [9]. Hydrolysate of 30 ml was dispensed into labelled 100 ml bottle and autoclaved at 121°C for 15 minutes and allowed to cool. Aseptically, the pH of the hydrolysate was adjusted to 5.5 (using NaOH and HCl) and inoculated with 0.3 ml of the S. cerevisiae suspension containing 2.9 x 106 cells/ml. In the control set-up however, the hydrolysates were not inoculated with S. cerevisiae. The bottles were tightly capped with polypropylene caps and sealed with the aid of an adhesive tape and incubated at 30°C at 100 rpm for 5 days in a shaking incubator for fermentation. The concentration of ethanol produced was determined at regular intervals of 12 hours for the 5 days’ fermentation duration using the gravimetric method. The experiment was carried out in triplicates and mean result used.
2.11. Chemical Analysis
- The chemical composition of the raw and the inoculated substrates was determined by proximate analysis using Van Soest method of analysis [12] for the determination of the fibre components, Neutral detergent fibre (NDF), Acid detergent fibre (ADF), and Acid detergent lignin (ADL), of the hydrolyzed and un-hydrolyzed substrates. These were used to estimate the cellulose, hemicellulose and lignin contents. Thus 1. NDF = Cellulose + Hemicellulose + Lignin + Minerals 2. ADF = Cellulose + Lignin + Minerals 3. Hemicellulose = NDF – ADF The means ± SD for each of the three replicates in every experiment were determined. Using a significance level of p < 0.05, an analysis of variance (ANOVA) was carried out and graphs (with standard deviation errors bars) plotted using Graph Pad Prism software (Version 6.0).
3. Results and Discussion
3.1. Figures 1 – 9 Represent the Graphs Plotted from the Data Obtained from the Laboratory Experiments
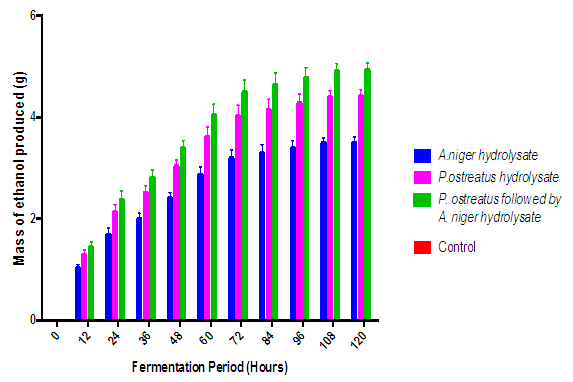 | Figure 4. Effect of fermentation of the various cassava peelings hydrolysate with S. cerevisiae on ethanol production (Control = hydrolysate with no Cerevisiae for fermentation process) |
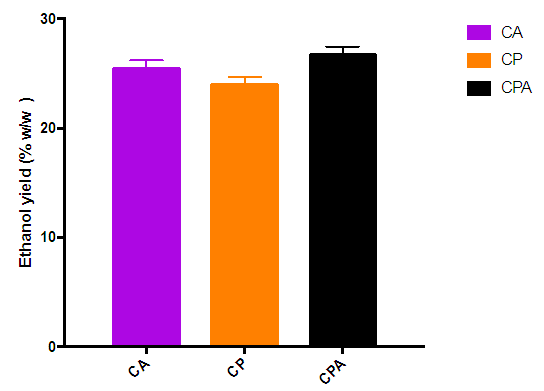
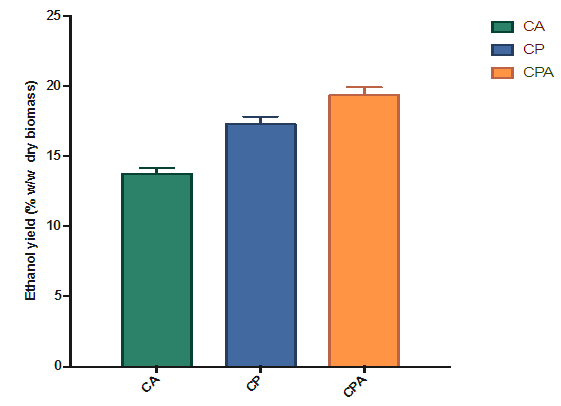 | Figure 6. Effect of the type of hydrolysis on percentage ethanol yield of dry biomass |
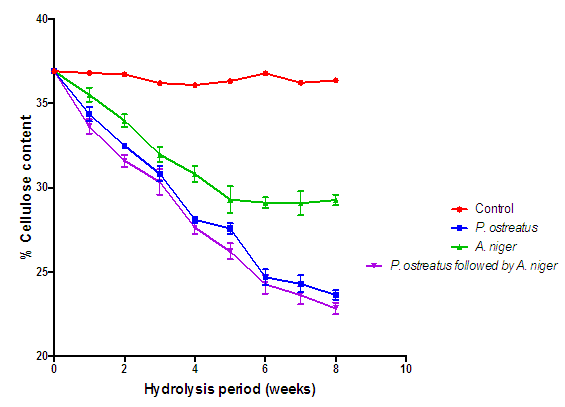 | Figure 7. Comparison of the effect of P. ostreatus k910 and A. niger MENAE1 hydrolysis on cellulose content of cassava peelings (Control=Cassava peelings not inoculated with fungi) |
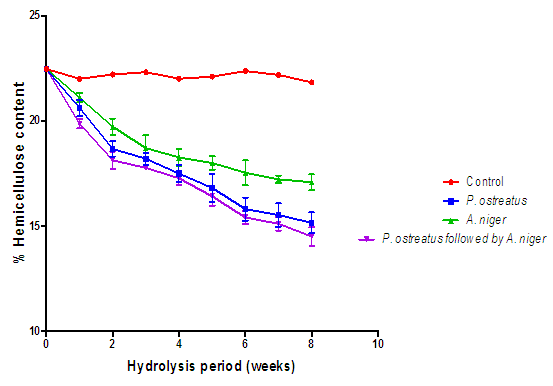 | Figure 8. Comparison of the effect of P. ostreatus k910 and A. niger MENAE1 hydrolysis on hemicellulose content of cassava peelings (Control=Cassava peelings not inoculated with fungi) |
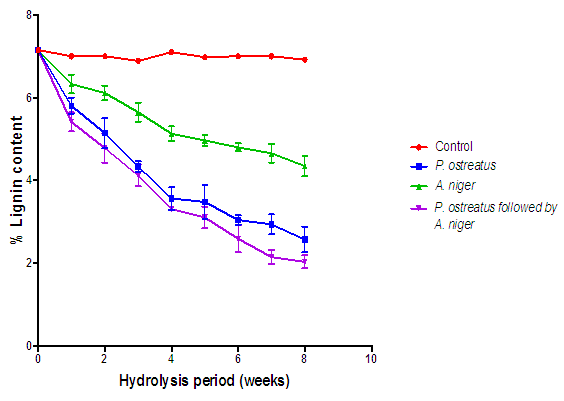 | Figure 9. Comparison of the effect of P. ostreatus k910 and A. niger MENAE1 hydrolysis on lignin content of cassava peelings (Control=Cassava peelings not inoculated with fungi) |
3.2. Effect of the Various Microorganisms Used for the Hydrolysis on Concentration of Sugar Produced
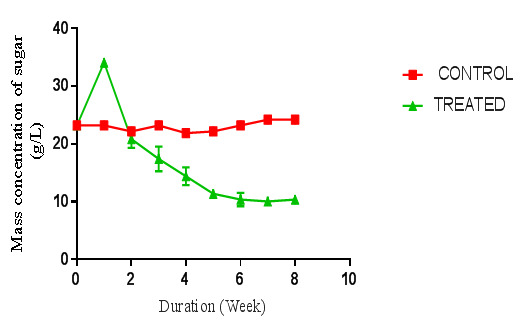
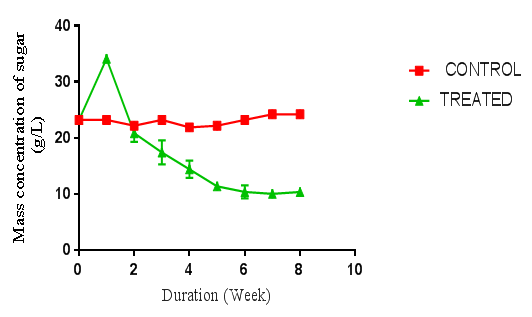
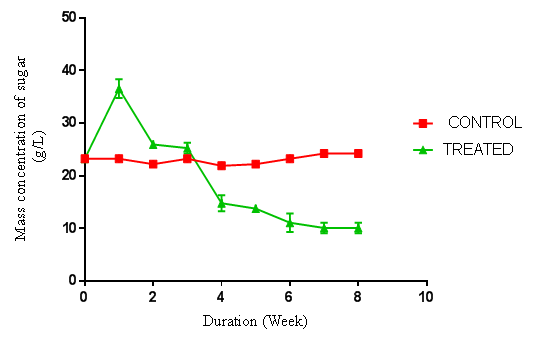
- The pretreatment method with initial milling of cassava peelings increased the surface area availability for extracellular enzymes activity. Also the size reduction and autoclaving of the substrates resulted in the release of celluloses and sugars. The total sugars of each raw material were determined to be lower than that obtained after autoclaving. Autoclaving for sterilization before fungal hydrolysis had an impact on the substrates resulting in increase of sugar content. This study confirms that physical pretreatments improved the cellulose content of the samples, and increased amount of fermentable sugar yield and ultimately bioethanol yield. The fungal treatments and hydrolysis increased the yields of sugars observed. When compared to individual fungal treatment, the combination of two fungi produced a higher sugar yield. A similar trend was reported in a work done by Kumar et al [13]. However, in their work, the substrates were rice straw, wheat straw and bagasse.Cassava peelings hydrolyzed with P. ostreatus produced higher concentration of 34.11 g/l of sugar as compared to 28.64 g/l obtained using A. niger MENAE1within one week of hydrolysis (Figure 1 and 2). This may be due to the synergistic nature of cellulases produced, indicating that the enzymes from P. ostreatus k910 may contain more of the components of the cellulase complex than A. niger. Also, P. ostreatus is one of the fungi found to have an elaborate higher exoglucanase and endoglucanase activities [14]. There was a sharp increase in sugar concentrations within the first week of hydrolysis of cassava peelings with the various fungi. This suggests that right after inoculating the cassava peelings, the microbes use the existing sugars present to catalyse the reaction and after which the fungi started using the available sugars for its growth. The highest fermentable sugar concentration (36.51 g/l) obtained after hydrolysis of P. ostreatus k910 hydrolyzed cassava peelings with A. niger MENAE1 for one week (Figure 3). This indicates that the fungi used the substrates as source carbon to break the cellulose into fermentable sugars. Subsequently the continuous decrease in fermentable sugar yield of cassava peelings after first week using the individual fungi or the combination of two fungi could be as result of the organisms feeding on the sugar produced. However, comparatively, the substrate hydrolyzed with the two fungi had relatively higher sugar than those hydrolyzed with either P. ostreatus or A. niger. This indicates that combining two fungi for hydrolysis improves fermentable sugar yield from cassava peelings. This is in agreement with the report of Olanbinwoninu and Odunfa that combinations of pretreatment boost the efficiency of reducing sugar yield from cassava peelings [15].
3.3. Fermentation of Hydrolysates of Cellulosic Waste Materials to Ethanol
- Ethanol production over the period of fermentation with S. cerevisiae progressively increased (Figure 4) with significant difference in ethanol concentration over the fermentation period of each hydrolysate. However, from the third day, there was no significant difference (p<0.05) in mean ethanol concentration. This could be a result of other by-products in the broth that might cause the inhibition of the yeast to ferment the available sugars in the broth. Also, high ethanol concentrations within the fermentation system can be major bottleneck for the S. cerevisiae, since this factor could slow down the fermentation process after the third day. It is also plausible that beyond the third day all the sugars that can be fermented by the S. cerevisiae would have been utilized. Theoretically, the maximum conversion efficiency of glucose to ethanol is 51% on a weight basis, which comes from a stoichiometric calculation of: 2 × (Molecular weight of Ethanol)/ (Molecular weight of Glucose) = (2 × 46)/ (180) = 0.51. In any case, S. cerevisiae consumes sugar for growth and production of other metabolic products and these, could be the reason for the shortfall in the yield.
3.4. Individual Fibre Components of Cassava Peelings Hydrolyzed with the Various Fungi
- The appearance of the fungal mycelia on the substrate after the first week was an indication that the degradation has commenced [16]. Generally, all the lignocellulose fractions of the substrates decreased significantly with respect to the hydrolysis time (Figures 7, 8 and 9). On the other hand, there were no significant changes (p > 0.05) in the controls. According to Irfan et al, degradation of lignocellulosic biomass results in weight loss [17]. It is interesting to note that the A. niger and P. ostreatus break down the various lignocellulosic fractions as carbon source for their nourishment. Progressive decrease in cellulose levels of all the hydrolyzed substrates are as a result of some extracellular fungal hydrolases (cellulases) breakdown the cellulose component of the substrates. These results further confirm those of Datta and Chakravarty [18] as well as that of Bentil et al [11]. Thus the breakdown of cellulose was a consequent of synergistic actions of some hydrolases (cellulases). P. ostreatus and A. niger have been reported to be sources of cellulases, hemicellulases and laccases, which are hydrolytic in nature and involved in the breakdown of complex carbohydrates into monomers (soluble sugars) [19]. Similarly, the hemicellulose content of all the substrates hydrolyzed with the various fungi decreased significantly (P < 0.05) with time. Nevertheless, this was not true for the controls, as they remained constant. The decrease in hemicellulose content of the various hydrolyzed substrates is because of hemicellulolytic enzymes (secreted by P. ostreatus and A. niger) activities on the substrates. This result confirms previous works reported by Alemawor et al [20] and Brimpong [21], where they reported 20-45% decrease in hemicellulose contents of some agro-wastes (lignocellulosic materials) hydrolyzed with P. ostreatus over time. It has been established that Pleurotus species produce enzymes with the capability of degrading a variety of β-(1,4) linked glucan substrates as well as glycosides [22]. Though a decreasing trend of hemicellulose content was seen throughout the hydrolysis duration, no significant decrease (p>0.05) was observed after the sixth week of hydrolysis, indicating that the optimum duration for hemicellulose degradation of cassava peelings with P. ostreatus is six weeks. At the end of six weeks (optimum fermentation period), hemicellulose content of cassava peelings hydrolyzed with P. ostreatus decreased by 39.99% after which there was no significant decrease. This could be a result of denaturation of secreted enzymes due to changes in pH or loss of moisture during the hydrolysis period. In congruence, Brimpong et al reported 41% decrease in hemicellulose content of corncobs hydrolyzed with P. ostreatus for duration of six weeks with no further decrease thereafter [21]. Melo et al. also showed that the enzyme activity dwindled with prolonged incubation [23].The control displayed no significant change (p>0.05) in lignin content. However, the lignin content of the substrates hydrolyzed with the various fungi was significantly (p<0.05) decreased over the hydrolysis period. Lignin hinders the biological breakdown of hemicelluloses and cellulose. The degradation of lignin was due to activities of extracellular lignin-degrading enzymes. These enzymes include lignin peroxidases (laccases) that oxidize aliphatic side chains as well as aromatic rings to produce compounds that are easily utilized by the fungi [24]. Therefore, the degradation of the lignin leads to making the cellulose more accessible [18].Lignin is one of the major constituents of cassava peelings which limit the use of such substrate in the bioethanol industry. Reduction in its content is of key importance to pretreatment step and greatly enhances second generation bioethanol production.
4. Conclusions
- Second-generation bioethanol was successfully produced from cassava peelings. P. ostreatus, A. niger and the combination of the two fungi were able to hydrolze the pretreated cassava peelings into fermentable sugars. The selected order of the two-stage biological pretreatment/ hydrolysis with P. ostreatus followed by A. niger significantly enhanced the concentration of fermentable sugars produced as compared to the individual fungi. The fermentable sugars produced were converted to bioethanol by S. cerevisiae. The hydrolysate obtained from hydrolysis of cassava peelings with P. ostreatus followed by A. niger gave the highest ethanol yield of 26.82% w/w (relative to sugar produced). Also the highest bioethanol yield relative to dry biomass obtained was 19.36% w/w when P. ostreatus followed by A. niger was used for the hydrolysis. Therefore, it can be concluded that the use of the two fungi in the hydrolysis process improved second generation bioethanol yield.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML