-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2017; 7(3): 53-57
doi:10.5923/j.ajee.20170703.01

Elemental Analysis of River, Marshes and Ground Water in Thi Qar Region, Iraq
Muhanad H. H. Alrakabi
Department of Physics, Al Mustansiriya University, Baghdad, Iraq
Correspondence to: Muhanad H. H. Alrakabi, Department of Physics, Al Mustansiriya University, Baghdad, Iraq.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

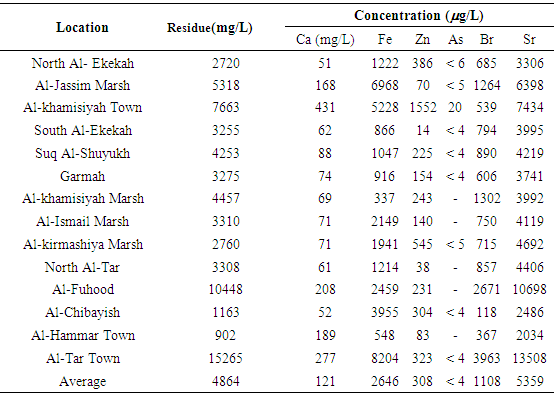
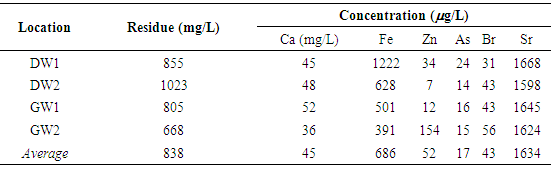
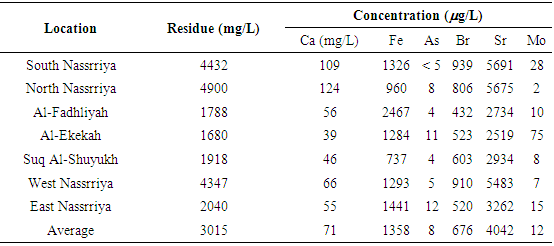
The elemental analysis of the samples collected from marshes water and river water in Thi Qar region of Iraq are done using the X-ray fluorescence technique (XRF). The water samples are collected from Euphrates River, Al- Hammar marshes, ground water and drinking water. The elements observed in various samples are 20Ca, 26Fe, 29Cu, 30Zn, 33As, 35Br and 38Sr. The 20Ca concentration in Euphrates river water constitutes ͂ 2.3% of the left over residue, a value similar to that observed in water from the marshes. The 38Sr concentrations in the Euphrates river water is in the range 2519-5691μg/L. The 38Sr concentrations in Euphrates River are similar to that observed in the marshes water. In Al-Hammar marshes, the concentration of 34As is estimated to be in general below 5 μg/L. The 35Br concentration in various samples collected from Al-Hammar marshes are in the average value =1108 μg/L. The 35Br concentration in various samples collected from Euphrates river are in the average value =676μg/L. The water from Euphrates river is not safe for use for drinking water supply due to high bromine content and the water from the drinking water schemes from the river needs to be monitored for the presence of bromate ions.
Keywords: X-ray fluorescence, River and Marshes water, Elemental Analysis, Drinking water contamination
Cite this paper: Muhanad H. H. Alrakabi, Elemental Analysis of River, Marshes and Ground Water in Thi Qar Region, Iraq, American Journal of Environmental Engineering, Vol. 7 No. 3, 2017, pp. 53-57. doi: 10.5923/j.ajee.20170703.01.
Article Outline
1. Introduction
- The Tigris and Euphrates rivers supply majority of the water into the marshes. The Euphrates traverses a distance of 2700 km, of which some 40% is in Turkey, 25% in Syria, and 35% in Iraq. The width of the Euphrates river ranges from 100-200 m, and the Tigris river from 150-350 m in the area of the marshes [1, 2]. The flow depths of the rivers are generally 5-15 m. The Euphrates river collects 84% of its flow in Turkey and 13% in Syria. It obtains no flow inputs within Iraq. Euphrates river water within Iraq is used for agricultural irrigation and municipal water supply. The core of the marshes in Thi Qar is centered in the area around the confluence of the Tigris and Euphrates rivers. It is typically divided into the two major areas: the Al-Hammar marshes, south of the Euphrates and the Central marshes between the twin rivers. Al-Hammar marshes are situated south of the Euphrates, extending from near Nassiriyah in the west to the outskirts of Basra on Shatt al-Arab in the east. Al-Hammar marsh area comprises 2800 km2 of permanent marsh and lake, expanding to over 4500 km2 during periods of seasonal and temporary inundation. Al-Hammar Lake, which dominates the marshes, is the largest water body in the lower Euphrates. It is approximately 120 km long and 25 km wide. Maximum water depth in the lake ranges from 2-3 m. Al-Hammar marshes are fed primarily by flooding and tributaries of the Euphrates river. Groundwater recharge is another source of replenishment. Local rainfall supplies very little water to the marshlands. The Iraqi marshes are largest wetland of unique ecosystem. The biotic community of marshes mostly includes plants and animals, which inhabit this rich environment. It is, therefore, very important that the remaining marshes be protected and that their health enhanced wherever possible. This is important for the surrounding environment and for the people who share this part of Iraq. In the last twenty years, Iraq has undergone two wars in 1991 and 2003, which have probably adverse repercussions on the Iraqi environment. These wars involved extensive burning, heavy bombing and shelling [3]. Also these wars took place in and around the study area under the present investigation, and caused considerable damage and pollution. To the best of our knowledge, no data are available in the scientific literature regarding the water contamination in south of Iraq after the recent Gulf war. The present study includes elemental analysis of the water sample collected from numbers of locations in the marshes and rivers in Thi Qar province in Southern Iraq. The elemental analysis has been performed using the X-ray Fluorescence (XRF) technique. The XRF technique is capable of detecting many elements simultaneously and has the advantage of low detection limit, which is further improved by analyzing the residue obtained after drying the water sample.
2. Materials and Methods
2.1. The Study Area
- The present study area is within Thi Qar (Nassiriyah) province and located in southern Iraq between the provinces of Waset to the north, Basra to the south, Maysan (Amarah) to the east and Muthanna to the west. It is located 350 km south of the capital Baghdad. The area of Thi Qar is 12900 km² with a population of 1.85 million. Thi Qar governorate has five districts: Nassiriyah, Suq Al-Shuyukh, Chibayish, Shatrah and Rafai. The water sample collection regions are shown in Fig. 1.
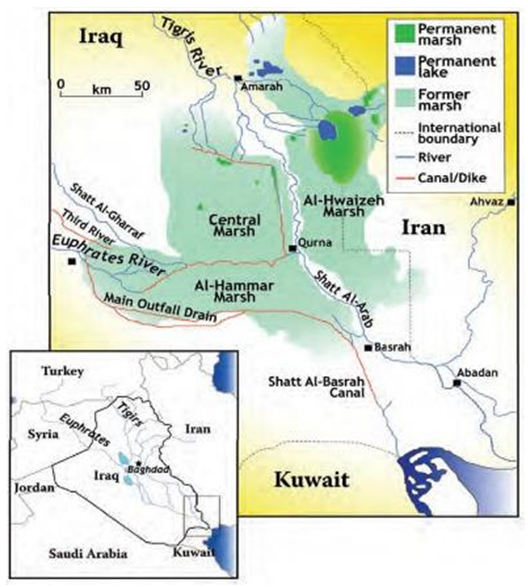 | Figure 1. Iraqi marshlands location map [4] |
2.2. Experimental Measurements and Evaluation Procedure
- The present study involved 26 locations from the Al-Hammar marshes, Euphrates rivers, drinking water and ground water from other locations. From each resource the water samples were taken in sterile plastic bottles under the surface of the water whenever possible. The bottles were labeled according to their sites and closed tightly. The water samples were filtered using normal filter paper (150 mm qualitative filter paper, whatman company, UK) and water samples were dried in disposable glass containers at ~ 70°C in an electric oven. About 100 ml of each of the water samples was used to obtain the residue. The residue was carefully scratched from the glass container and the remain sticking to the glass was removed using citric acid. The water residue samples were weighed and pressed into pellets of thickness 40-150 mg/cm2. The elemental analysis of the samples was performed using the XRF spectrometer shown in Fig. 2. The exciter source consisted of a 3 kW long-fine-focus 42Mo-anode X-ray diffraction tube along with a 4 kW X-ray generator procured from Pan Analytic, the Netherlands.
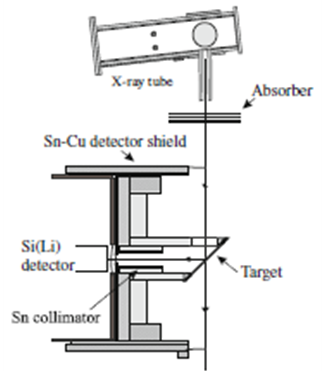 | Figure 2. The XRF set up used in the present work |
3. Results and Discussion
- Typical water residue spectra from water samples collected from various places are shown in Figs. 3-5. The spectra show peaks of the K X-rays of 20Ca, 26Fe, 29Cu, 30Zn, 33As, 35Br and 38Sr.
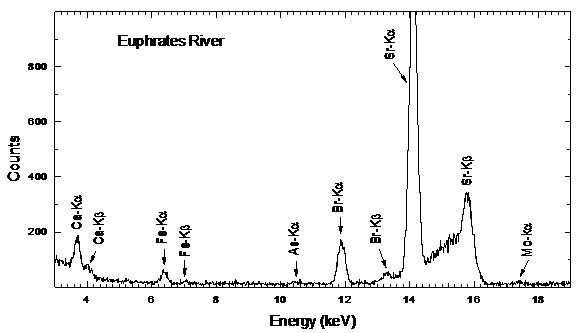 | Figure 3. Typical spectra from residues of water samples taken from Euphrats River |
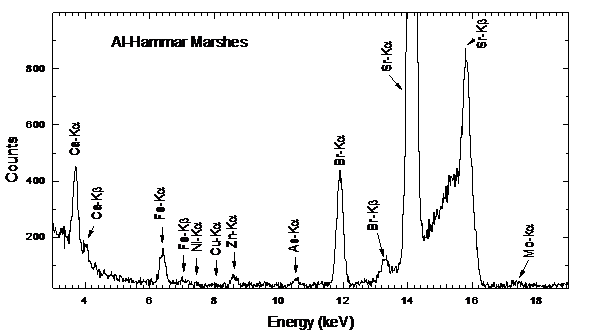 | Figure 4. Typical spectra from residues of water samples taken from Al-Hammar Marshes |
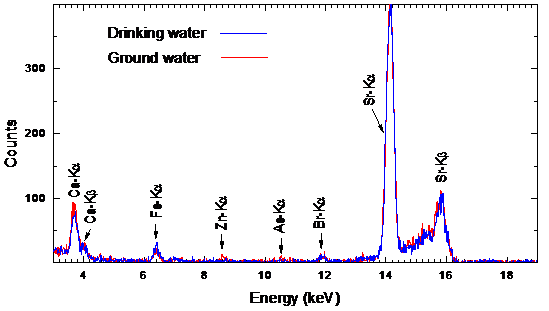 | Figure 5. Typical spectra from residues of Ground water and drinking water samples taken from Thi qar region |
|
|
|
4. Conclusions
- From the present study, it is concluded that water from Euphrates river not safe for use for drinking water supply due to high bromine content and the water from the drinking water schemes from the river needs to be monitored for the presence of bromate ions. The drinking water and ground water samples from Thi Qar region show similar concentrations of 20Ca, 35Br and 38Sr. The drinking water samples have been collected from water supply schemes based on the river water. The concentration of 38Sr is ~ 1650 μg/L, which is much higher than the permissible limit of 70 μg/L [10, 11] throughout the study area. Very few values above 1000 μg/L have been reported for waters, for instance, 2980 μg/L for an Australian river [12, 13]. The high 38Sr contents in study area water samples were likely related to the dissolution of Calcium-sulphates such as gypsum. It is well known that Sr2+ easily replaces Ca2+ in the crystalline network of several mineral phases, so that these ions are usually well correlated. The samples of drinking water supply show higher concentration of 26Fe and 30Zn, which are likely to be linked to the water pumps and pipes used in the system. Traces of 33As have also been observed in the ground water. It is recommended that drinking water sources in the study area should be routinely monitored to ascertain its suitability for drinking and other purposes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML