Ahmad Kholil, Darwin Rio Budi Syaka, Eko Arif Syaefuddin, Rizky Marrapelico
Department of Mechanical Engineering Education, State University of Jakarta, Indonesia
Correspondence to: Ahmad Kholil, Department of Mechanical Engineering Education, State University of Jakarta, Indonesia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The problem in small and remote islands is limited freshwater and waste management. One solution to solve the fresh water and waste problem is by using waste as an energy source to convert sea water into fresh water (desalination). To this end, the purpose of this research is to design and build seawater desalination apparatus that uses waste as a source of energy. This research was conducted by making the tool then testing. Testing is done by using four different types of waste: coconut shells, twigs, plastics and mix wastes with four different variations sea water volume: 123 liters, 103 liters, 74 liters and 53 liters. Burning temperature is measured using a thermocouple, salinity was measured using a salinity meter, and the acidity of the water was measured with litmus paper. The results of this study are the dimensions of the tool according to the scale of households in the islands. Dimensions of 100 cm (length) x 80 cm (width) x 100 cm (height) with the volume of the burning chamber can accommodate 2 kg of waste. Sea water volume increase causing the volume of fresh water and salt levels also increased. The highest volume of water was obtained using plastic wastes. Lowest salt levels obtained when using wood wastes. A significant difference in the production of freshwater was seen as a result of using different types of waste.
Keywords:
Desalination, Waste, Remote island
Cite this paper: Ahmad Kholil, Darwin Rio Budi Syaka, Eko Arif Syaefuddin, Rizky Marrapelico, Desalination Sea Water Using Waste as a Source of Energy for Small and Remote Island, American Journal of Environmental Engineering, Vol. 6 No. 6, 2016, pp. 188-195. doi: 10.5923/j.ajee.20160606.05.
1. Introduction
Indonesia is a maritime country that consists of a stretch of islands composed of Sabang to Merauke there are also hundreds of small islands that have a problem with fresh water. The background of this issue by many factors, ranging from the lack of infrastructure, access to transportation to reach the island as well as many other things that lead to much freshwater crisis on small islands in Indonesia. Desalination means the separation of fresh water from salt water. The method used in the desalination process is called brine. In the separation of fresh water from salt water, there are several desalination process technologies that have been widely known, among others, the process of distillation / evaporation process technology with a membrane, the ion exchange process etc. Desalination tool that we have designed is a tool desalination by using methods of distillation (evaporation). In the distillation process, seawater is heated to evaporate sea water and then the resulting water vapor is condensed to obtain fresh water. This process produces pure water better compared with other processes. The heat energy is a major component in the process of distillation here we replace it by using the waste, this thing we do to help the preservation of the island. The designed tool to desalinate seawater into fresh water by using waste as a source of heat energy is expected to help meet the needs of freshwater adequate for the entire population of the islands. Trash essentially contains energy. In the organic waste in the form of the rest of the plant, the energy came from the sun captured by green plants through photosynthesis. Non-organic waste is plastic contains energy derived from fuel oil, coal and gas used in chemical synthesis process are simple becomes complex chemical substances. Energy in organic waste / non-organic, either in the form of residual or leftover plant materials such as synthetic chemical can be released again by burning. The energy released can be used to heat the seawater desalination process into fresh water. Based on this research aims to design and build seawater desalination apparatus with the use of waste as a source of energy as a solution to the crisis freshwater environment-friendly to the needs of small and remote islands. Therefore, seawater desalination apparatus with the use of waste as an energy source that is easy and inexpensive to manufacture, simple in operation and maintenance, as well as fresh produce pure water.
2. Desalination Theory
Desalination is the separation of fresh water from salt water. The method used in the desalination process is called brine. In the separation of fresh water from salt water, several distillation technologies are widely known: the processing technology with a membrane, the ion exchange process, the process of distillation/evaporation and freezing [12].Selecting which one is the appropriate technology depends on many factors including the processing location, the sea water quality, what the resulting water is going to be used for. Given the increasing demand for fresh water by both human and industry, every country needs to provide inexpensive freshwater despite the cost for the higher procurement of energy sources [12]. Producing fresh water from seawater by freezing relies on the principle that the individual ice crystal structure can not accommodate salt. Therefore, saline solution during freezing, ice crystals will reject salts, so that the ice crystals can be separated from the concentrated solution of salt water, and then the ice crystals can be melted to produce pure water [6]. Research methods of desalination freezing for commercial purposes are generally directed to be used for remote areas, cold areas and for small and remote communities [3] or special purposes, for example the water treatment in mining [6]. However, freezing desalination process has not been fully developed or commercialized for seawater applications, because it offers no significant advantages over other methods of desalination. The main reasons for this are the high costs, no significant savings [6] and the produced water has a low pH, calcium, and alkalinity which requires further processing for safe consumption [3], so the system is not suitable to be applied in small and remote islands in Indonesia minimal infrastructure facilities.In the desalination process of ion exchange, a simple power supply is used to generate a 3.0 V bias potential microelectrochemical cell consists of two microchannels spanned by a single bipolar electrode (BPE) to promote the oxidation of chloride and the electrolysis of water at the poles BPE. This results in the depletion zone of ions and electric fields into branching microchannel, consequently producing desalted water [1]. Despite offering high efficiency, the microchannel application is high-cost, complex and is not suitable for Indonesia conditions. Salt water desalination membranes are commonly used in desalination technology system with Reverse osmosis (RO). Reverse osmosis (RO) is done by giving a higher osmotic pressure on the seawater exceeds the pressure on the solution with a low concentration (freshwater). Thus, the water flows in the opposite direction of the natural flow across the membrane, leaving the dissolved salts. No heating or phase change is required [2]. RO desalination technology is the most successful process for the commercial production fresh water from sea water in large volume [4, 2]. However, this technology is still partially dependent on import components such as the high-pressure pump RO membrane. Such dependence results in high production cost [11]. The simplest and widely known seawater desalination process is the distillation process. In principle, distillation is a way to get clean water through the distillation process. Distillation is a mixture of heat transfer, evaporation and condensation heat process. If the water continues - there will be a constantly heated evaporation process. This steam in contact with a cold surface, there will be condensation on cold surfaces such. In the distillation process are taken only condensate water, germs and bacteria will die by the heating process, and dirt will settle to the bottom basin. Solar energy is an alternative energy that can be used in the heating process [10]. Using solar energy means, however, that the distillation process is highly dependent on the weather. In addition, the manufacturing and maintenance of solar collectors are complicated and high cost which is not suitable for the population on the islands. There has been a study that created a simple water distillation equipment which is designed only to use local components and low cost, making it affordable for most people [5]. This technology, however, relies on electric heaters as a source of energy. Based on the above discussion, the design tools are simple salt water desalination in the manufacture and maintenance and is less costly seawater distillation process using direct thermal energy sources. Based on preliminary studies conducted in the thousand islands, rubbish is widely available and cost nothing for a source of energy. Such rubbish is produced by households. The rubbish can also be found in open water which originated from Jakarta Bay. The use of waste as a source of energy in desalination to convert salt water to freshwater is therefore economically efficient and at the same time addresses trash problem in kepulauan seribu. Research on the use of waste as a source of energy to convert sea water into fresh water is limited. One such research was carried out by Udono and Sitte (2008). They created seawater desalination model powered by burning waste using a dynamic system approach. However, this study was limited to mathematical models and to scale waste in urban areas. Therefore this paper proposes a prototype for saltwater distillation apparatus that use household waste as the source of energy and designed to suit the need of people living in Indonesian small islands.
3. Materials and Method
We conducted a laboratory experiment to test different types of waste to determine how much water can be produced from the distillation process. The dimension of the furnaces was tailored to meet household scale needs. The furnaces were 100 cm (length) x 80 cm (width) x 100 cm (height) with a burning chamber that can accommodate 2 kg of waste. This size is based on the observation in the community that the average amount of waste generated each household is around 1.75 kg with varied types of waste. The design of the distillation equipment is shown in Figure 1.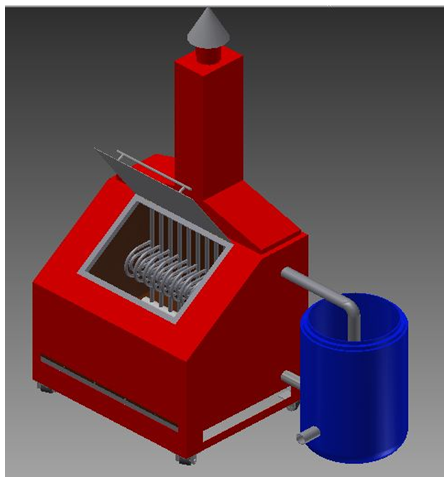 | Figure 1. Distillation furnace model |
In the distillation process, seawater is heated to evaporate sea water and then the resulting water vapor is condensed to obtain fresh water. This process produces a very high fresh water purity level compared with other processes. The sea water boils at 100°C at atmospheric pressure, but may boil below 100°C when the pressure is lowered.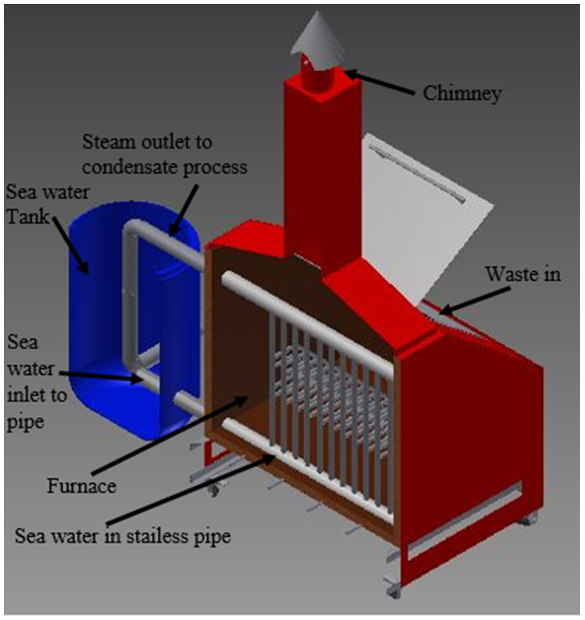 | Figure 2. Section cut of furnace |
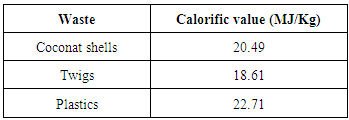
The work process of furnace begins by entering sea water in a tank. The water in the tank will go into stainless steel pipe up to the level of the water in the tank. Waste is put into the furnace, and then burned and closed the door so the smoke come out only at the chimney. After some time the sea water begins to boil and evaporate go to the top of the pipes. Exit pipe connected to the tank, so that the steam is condensed by seawater that there are reservoirs. After the steam is condensed, the distilled water out of the pipe and ready to use.In the process of testing was conducted using a furnace that does not use a blower to aid the circulation of burning in the furnace so that burning occurs naturally.Testing on distillation furnace based on the volume of sea water to determine the salinity value characteristics in each type of volume. In this research, four times the test with different water volumes, including:a. 123 liters of sea water; b. 103 liters of sea water; c. 74 liters of sea water; and d. 53 liters of sea water.While the types of waste used in this experiment consist of: a. coconut shells b. mix wastes;c. plastics; andd. twigsThe potential of waste that are abundant for future energy solutions can be categorized as the use of ecologically-friendly and abundant existence in the world, especially in Indonesia. Each type of waste has different levels depending volatile. Volatile compounds which evaporate contained in the waste types that affect how reactive products of combustion. The content of fixed carbon and volatile matter very influential on the calorific value of each type of waste. The calorific value of the waste used in this study as follows:Table 1. The calorific value of waste
 |
| |
|
The volume of waste entering the combustion chamber is 4 kg. Based on the waste calorific value and volume of waste entering the combustion chamber, the heat generated from the burning furnace shown in Figure 3.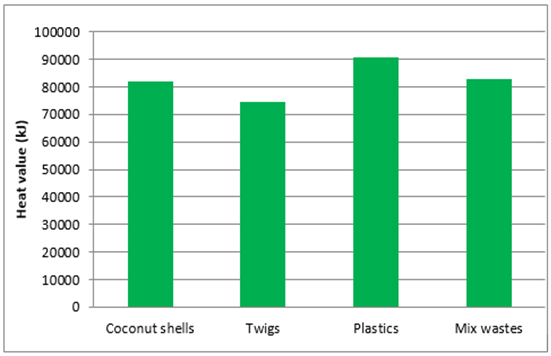 | Figure 3. Heat generated from each waste |
The calorific value of the waste generated is used as a reference to the need to heat the furnace heat pipes in the distillation furnace.
4. Result and Discussion
4.1. Testing Furnaces
Testing furnace aims to determine the characteristics of the calorific value of each type of waste. Tests carried out using fuel type of coconut shell, wood sticks, plastic and mixed. Each fuel tested four times and averaged the data retrieved. Testing is done by measuring the temperature of the water in the tank, chamber furnaces, chimneys, and water is produced.
4.1.1. The Test Results Using Coconut Shells
Figure 4 shows that the highest temperature in the furnace is equal to 339°C and the lowest temperature is 168.5°C with an average burning time 28 minutes 17 seconds and produce high levels of opacity as much as 0.6%.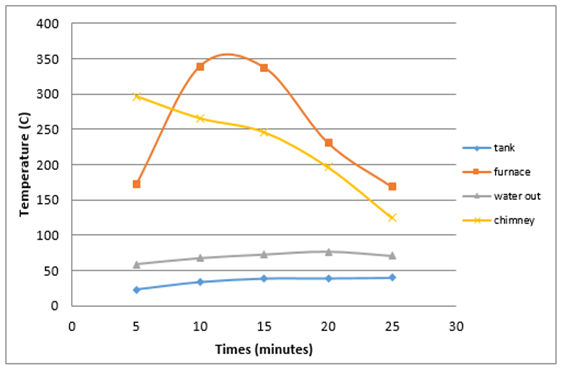 | Figure 4. Average heat produced by burning coconut shells |
 | Figure 5. Coconut shells |
4.1.2. The Test Results Using Twigs
Figure 6 shows that the highest temperature in the furnace is equal 239,75°C and the lowest temperature is 91.75°C with an average burning time 28 minutes and 27 seconds and produce high levels of opacity as much as 1.4%. 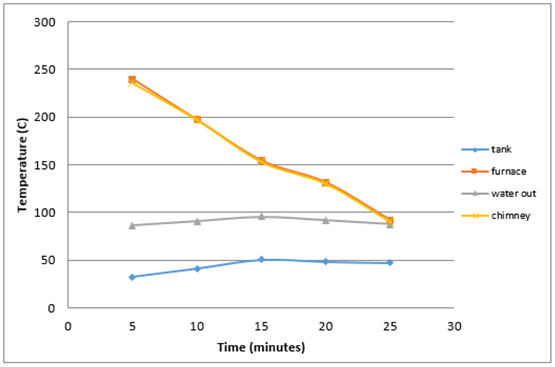 | Figure 6. Average heat generated by burning twigs |
 | Figure 7. Twigs |
4.1.3. The Test Results Using Plastics
Figure 8 shows that the highest temperature in the furnace is equal 856°C and the lowest temperature is 792°C with an average time of 18 minutes 38 seconds burning and produce high levels of opacity as much as 66.07%.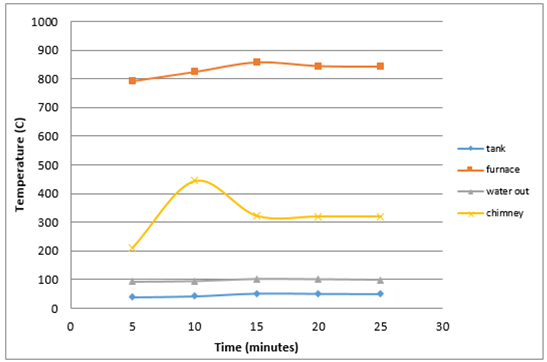 | Figure 8. Average heat generated by burning plastics |
 | Figure 9. Plastics |
4.1.4. The Test Results Using Mix Wastes
Figure 10 shows that the highest temperature in the furnace is equal 339,75°C and the lowest temperature that 209.25°C with an average burning time 23 minutes and produce high levels of opacity as much as 6.7%.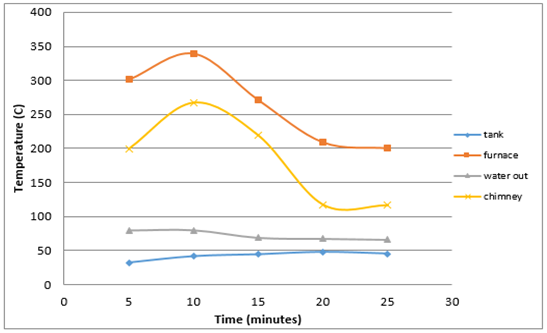 | Figure 10. Average heat generated by burning mix wastes |
4.2. Comparison between the Waste to the Temperature
This comparison to find out the temperature differences resulting from each type of waste that is burned so as to facilitate in the selection of proper waste for incineration in the distillation process.Figure 11 shows that the temperature generated by the burning of plastics is very high up to 858°C. This is influenced by the calorific value of each of the different types of waste, where the waste plastic has the highest calorific value compared with calorific values of other types of waste.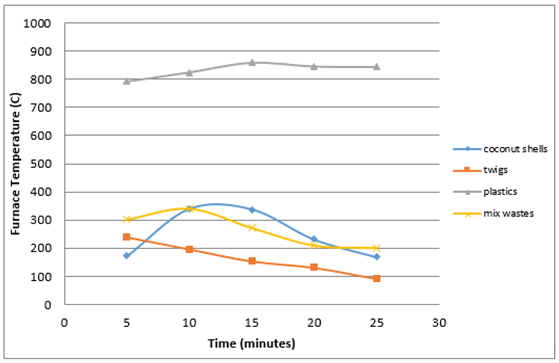 | Figure 11. The temperature of each wastes |
Burning time on each wastes varies depending on the types of wastes, in figure 12, coconut shells and twigs tend to have a burning time longer than the plastic waste and mixed, this happens because at the time the waste is burned in addition to produce a flame great but also alter the coconut shells and twigs into the coals, so as to maintain the temperature inside the furnace room last longer, while the plastic waste when burned produces very high temperatures but with a relatively short time because of changes in the form of plastic into liquid, so it is not able to maintain the temperature inside the furnace.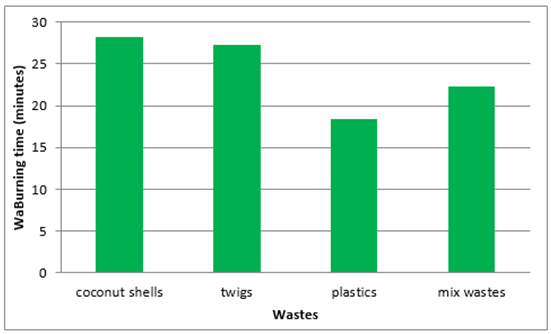 | Figure 12. Burning time of each wastes |
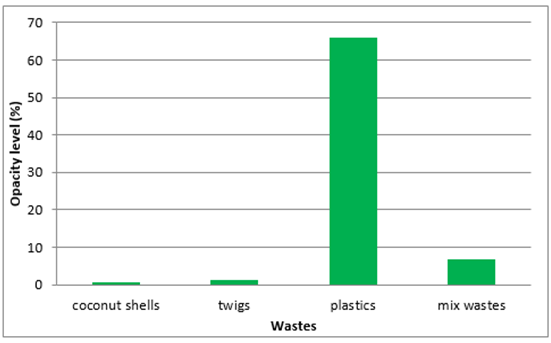 | Figure 13. Opacity levels |
Levels of opacity of the exhaust gases indicate that the burning of plastic waste that generate the greatest levels of opacity, and the lowest is the burning of coconut shells. This can occur because of a plastic material made from materials that contain a lot of chemicals while the coconut shells and twigs wood is a natural material so that when burned produce little pollution. Combustion is influenced by three things, fuel (wastes), fire, and air. In this test, the burning is done naturally without the aid of a blower that greatly affects weather around the burning rate.
4.3. Salinity Analysis to the Water Volume
Testing salinity of the water volume is done by testing furnace that is equipped with pipes filled with water to evaporation. Testing is done by incineration in furnaces with a variation of incoming water volume 123 liters, 103 liters, 74 liters and 54 liters. While the types of waste that used coconut shells, twig, plastic, and mix waste.
4.3.1. Burning Analysis of 123 Liters Sea Water
On volume of 123 liters of space in the pipeline for a little evaporation so that salt come bottom of the steam and the measurement salinity results showed 27 ppt. Burning using coconut shells and burning using sticks and mixed waste plastic is quite different because the salt content in the furnace temperature using coconut shell trash burning takes 25 minutes and reach the hot spots while temperatures reached 212.6°C wood sticks and plastic waste just 20 minutes and reached the point 432,5°C furnace heat.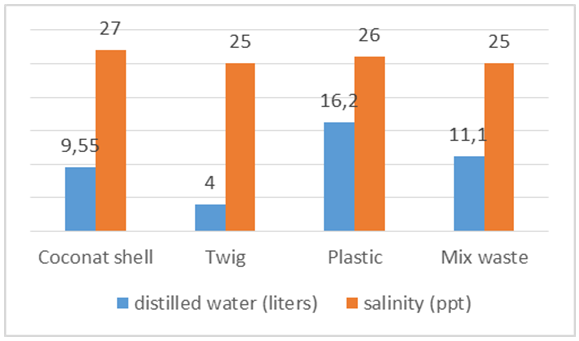 | Figure 14. The average salinity and water produced from sea water 123 liters |
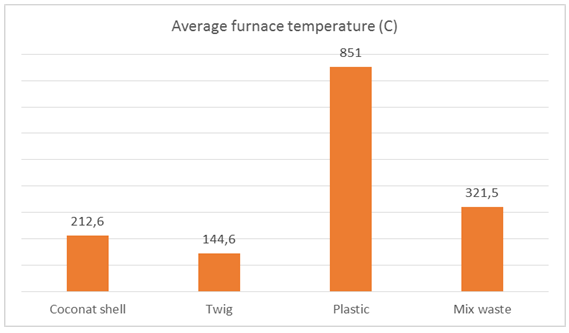 | Figure 15. The average furnace temperature of 123 liters of sea water |
4.3.2. Burning Analysis of 103 Liters Sea Water
On the volume of sea water 103 liters of space in the pipe for evaporation bit so that salt come bottom of the steam so that the salt content is high enough but by using a water volume of 103 liters, salinity decreases compared to using a water volume of 123 liters is because there is an evaporation chamber that compared with the volume of water 123.In Figure 16 the burning using coconut shells and burning using mixed waste wood sticks and plastic is quite different from its salinity due to the temperature of the furnace using waste coconut shell require burning time 25 minutes and reach the hot spots temperature reaches 306°C while waste sticks and plastic just 20 minutes and reached a furnace heat 295°C.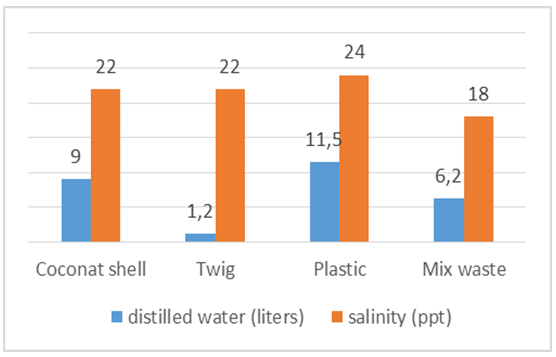 | Figure 16. The average salinity and water produced from sea water 103 liters |
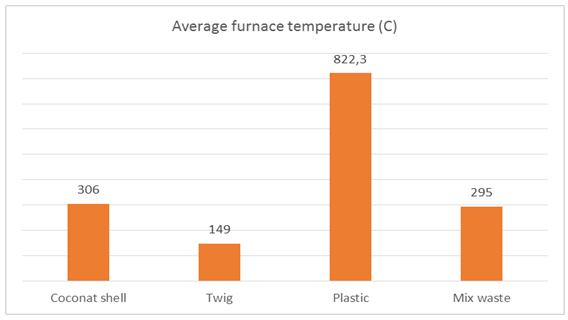 | Figure 17. The average furnace temperature of 103 liters of sea water |
4.3.3. Burning Analysis of 74 Liters Sea Water
In figure 18 seen the influence of the kind of waste that is burned on the graph salt content and the volume of distilled water to volume of 74 liters of water less salt content due to the evaporation chamber larger pipe so that the salt does not come into the flow of evaporation it causes the salt content is low. Refuse coconut shell, reaching 17 ppt salinity and water volume produced 2.7 liters. While the use of mixed waste plastic and sticks reach 12 ppt to generate less water results from distillation processes due to the process of using the mixed waste in the furnace temperature to 250°C and in the process of burning waste just 20 minutes while the coconut shell achieve 278.8°C and heat in the burning process up to 25 minutes so that the water volume reached at using coconut shell litter more distilled water.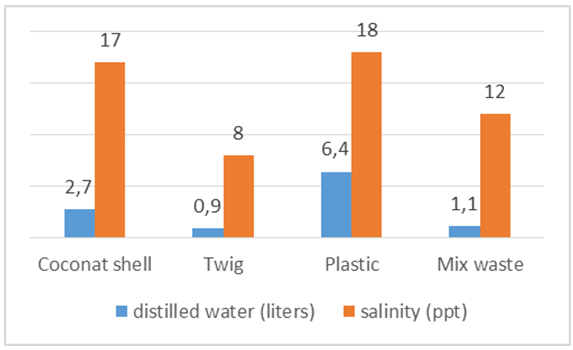 | Figure 18. The average salinity and water produced from sea water 74 liters |
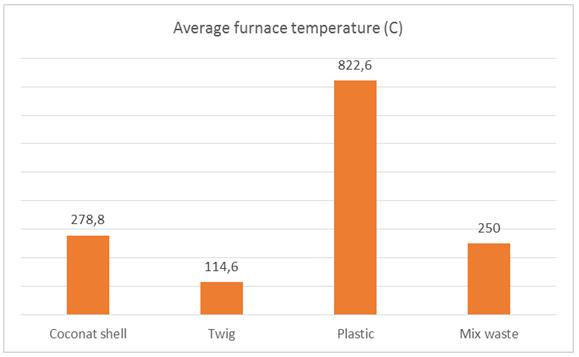 | Figure 19. The average furnace temperature of 74 liters of sea water |
4.3.4. Burning Analysis of 53 Liters Sea Water
In figure 20, using coconut shell litter at 16 ppt salinity water volume produced 0.75 liter, while using mixed waste plastic and sticks reach 12 ppt to generate less water results from distillation processes due to the process of using mixed waste temperatures 255,2°C in the furnace and in the process of burning waste just 20 minutes while the coconut shell achieve 200,6°C and heat in the burning process up to 25 minutes so that the volume of water that is obtained coconut shell litter produced more water. Refuse wood sticks reach 6 ppt to produce 0.68 liters of water volume.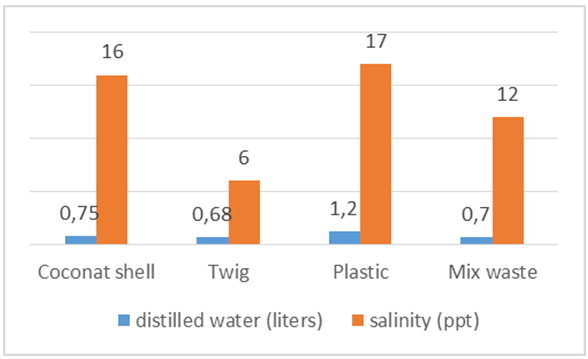 | Figure 20. The average salinity and water produced from sea water 53 liters |
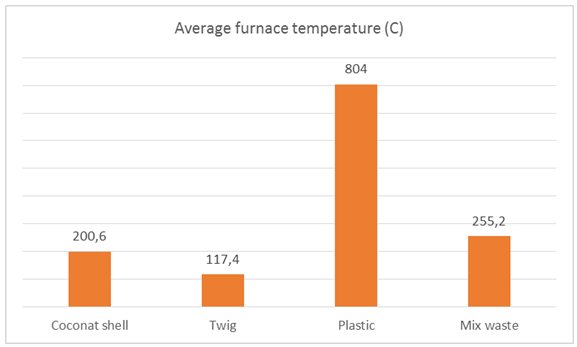 | Figure 21. The average furnace temperature of 53 liters of sea water |
4.4. Influence the Waste to Salt
The influence of the type of waste to salt is performed to determine whether each type of waste can affect the outcome of the salt content of the water distillation. Can be seen from the graph where the highest levels found in testing ppt on junk shell with water level at 123 liters. This is due to the evaporation of the water volume at the point of 123 liters, only 1.7 Liter so little room for evaporation so that salt come bottom by a little steam and due to the length of time the burning of a coconut shell.Can be seen on the figure 11 that the lowest point of salinity on experiments with litter wood sticks at 6 ppt on trial 53 liter volume of 2.58 liter due to the evaporation chamber so that the salt does not come into the flow of evaporation it causes the salt content is low.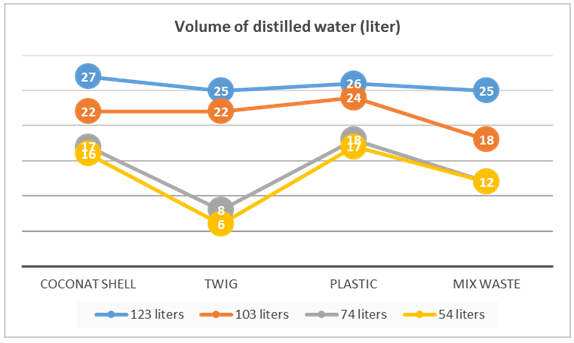 | Figure 22. Average volume of distilled water |
4.5. Burning Efficiency
Burning efficiency analysis can be done to determine what percentage of the heat absorbed by means of distillation by each type of waste and volume of water is different.Figure 23 shows that the burning of 123 liter volume tends to be more efficient than burning in other volume, it is due to the heated volume is directly proportional to the volume of burning. Burning at the volume of 54 liters of water tend to have low efficiency. Efficiency is also affected by a series of heating pipes, the more the greater the heating pipe cross section, the heat is absorbed more and more, it can improve burning efficiency results. The greatest efficiency is the type of plastic waste, this is because the temperatures resulting from the burning of plastic waste is very high at 858°C resulting in a rapid circulation of the distillation process is able to produce more water. It is very affect on the efficiency of burning waste plastic types.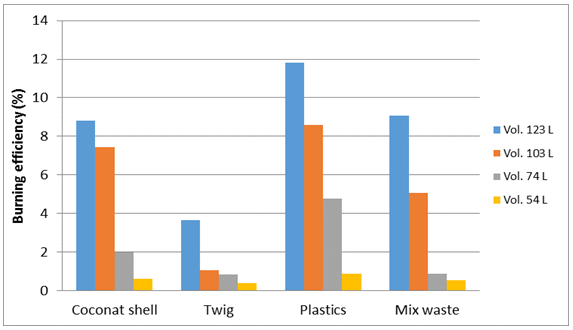 | Figure 23. Burning efficiency |
5. Conclusions
a. The more the volume of water used to produce the volume of water that much but the resulting salinity is also higher. From the test results show that the volume of sea water to produce 123 liters of fresh water are more when compared with the volume of water other testing. This is because the volume of water that can be evaporated in the pipes more distillation.b. The volume of water produced the highest obtained when using plastic waste because the burning temperature generated the highest when compared with other types of wastes such as coconut shells, sticks and mixed waste.c. Lowest salt levels obtained when using this type of waste in trash timber for heat stable sticks and twigs wood calorific value is smaller when compared with the three types of waste.d. There is a significant relationship between the type of waste by volume of water produced results based on test results to the freshwater types of waste generatede. The highest burning efficiency obtained in testing with a volume of 123 liters of water. This is because the volume of water is more vaporized in the cheek-pipe distillation.f. Using plastic waste led to increased levels of opacity that pollute the environment. Therefore the use of natural waste such as twigs and coconut shells are better for this furnace is used in small and remote islands.
ACKNOWLEDGEMENTS
Authors are highly thankful to Kementrian Riset Teknologi dan Pendidikan Tinggi Republik Indonesia for funding this research.
References
| [1] | Knust. Kyle N., Hlushkou. Dzmitry, Anand. Robbyn K., Tallarek. Ulrich, dan Crooks. Richard M., 2013, Electrochemically Mediated Seawater Desalination, Angew. Chem. Int. Ed. 2013. |
| [2] | Khawajia. Akili. D., Kutubkhanaha. Ibrahim K., Wieb. Jong-Mihn, 2008, Advances in seawater desalination technologies, Desalination 221 (2008) 47–69. |
| [3] | Mahdavi. Mokhtar, Mahvi. Amir Hossein, Nasseri. Simin, Yunesian. Masoud, 2011, Application of Freezing to the Desalination of SalineWater, Arab J Sci Eng (2011) 36:1171–1177. |
| [4] | Mathioulakis. E., Belessiotis. V., Delyannis. E., 2008, Desalination by using alternative energy:Review and state-of-the-art, Desalination 203 (2007) 346–365. |
| [5] | Oyawale F. A., Odior, A.O., Ismaila M., 2010, Design and Fabrication of a Water Distiller, Journal of Emerging Trends in Engineering and Applied Sciences (JETEAS) 1 (2): 169-174. |
| [6] | Shonet R.D.C., 1987, The freeze desalination of mine waters, J. S. At,. Inst. Min. Metal/., vol. 87, no. 4. Apr. 1987. pp. 107-112. |
| [7] | Udono. Ken, Sitte. Renate, 2008, Modeling seawater desalination powered by waste incineration using a dynamic systems approach, Desalination 229 (2008) 302–317. |
| [8] | Irianto, eko w, dkk., Instalasi Pengolahan Air Sangat Sederhana., No37 Tahun III-KW 1996, Puslitbang Pengairan. |
| [9] | Walangare, dkk., Rancang Bangun Alat Konversi Air Laut Menjadi Air minum Dengan Proses Destilasi Sederhana Dengan Menggunakan Pemanas Elektrik., Teknik elektro, Unsrat, 2003. |
| [10] | Astawa. Ketut, Sucipta. Made, Negara. I Putu Gede Artha, 2011, Analisa Performansi Destilasi Air Laut Tenaga Surya Menggunakan Penyerap Radiasi Surya Tipe Bergelombang Berbahan Dasar Beton, Jurnal Ilmiah Teknik Mesin Cakra M, Vol. 5 No.1. April 2011 (7-13). |
| [11] | Indriatmoko. Robertus Haryoto, Herlambang. Arie, 1999, Pengelolahan Air Asin dengan Sistem Osmosis Balik, Badan Pengkajian dan Penerapan Teknologi. hlm.138. |
| [12] | Said. Nusa Idaman, 2003, Aplikasi Teknologi Osmosis Balik Untuk Memenuhi Kebutuhan Air Minum Di Kawasan Pesisir Atau Pulau Terpencil, J.Tek.Ling. P3TL-BPPT.4(2):15-34. |

























 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML