-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2016; 6(5): 129-139
doi:10.5923/j.ajee.20160605.01

Dynamic Model for Anaerobic Digestion of Sewage Sludge Considering Haldane Kinetics and Wall Growth Factor
Pooja Sharma, Uttam Kumar Ghosh, Amiya Kumar Ray
Department of Polymer and Process Engineering, Indian Institute of Technology Roorkee, Saharanpur Campus, Saharanpur, (UP), India
Correspondence to: Pooja Sharma, Department of Polymer and Process Engineering, Indian Institute of Technology Roorkee, Saharanpur Campus, Saharanpur, (UP), India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Simulation studies using dynamic model equations based on Haldane kinetics were performed to predict the optimized process parameters for anaerobic digestion of sewage sludge. Stability analysis of digester under high loading rate conditions was carried out considering wall growth factor (WGF). Initial and optimized process conditions for microbial concentration 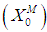 and inhibition constant
and inhibition constant 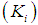 were taken from batch studies. Simulation was performed by varying the values of
were taken from batch studies. Simulation was performed by varying the values of 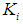 at four constant values of
at four constant values of 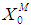 . Poor process performance in terms of COD removal has been observed at lower values of
. Poor process performance in terms of COD removal has been observed at lower values of 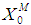 , even at higher values of inhibition constant
, even at higher values of inhibition constant 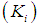 for continuous process. Maximum COD removal efficiency was observed by operating the digester at
for continuous process. Maximum COD removal efficiency was observed by operating the digester at 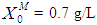 and
and 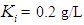 . COD removal efficiency increased from 12% to 48% at higher loading rate of
. COD removal efficiency increased from 12% to 48% at higher loading rate of 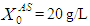 and retention time of 15 day when WGF was included in the model equation. Under similar conditions COD removal efficiency decreased from 40% to 6.6% when the loading rate
and retention time of 15 day when WGF was included in the model equation. Under similar conditions COD removal efficiency decreased from 40% to 6.6% when the loading rate 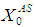 was increased from 40g/L to 45 g/L, however, increase in retention time up to 20 day resulted in re-stabilized process conditions.
was increased from 40g/L to 45 g/L, however, increase in retention time up to 20 day resulted in re-stabilized process conditions.
Keywords: Anaerobic digester, Sewage sludge, Wall growth factor, Haldane model
Cite this paper: Pooja Sharma, Uttam Kumar Ghosh, Amiya Kumar Ray, Dynamic Model for Anaerobic Digestion of Sewage Sludge Considering Haldane Kinetics and Wall Growth Factor, American Journal of Environmental Engineering, Vol. 6 No. 5, 2016, pp. 129-139. doi: 10.5923/j.ajee.20160605.01.
Article Outline
1. Introduction
- Generation of wastewater and sludges from various municipal and industrial activities require stabilization of organic pollutants prior to disposal into the environment or for further reuse. Biological wastewater treatment processes, anaerobic digestion, in particular mostly adopted for treatment of high organic strength wastes such as municipal sludges and industrial effluent (Bello-Mendoza et al., 1998; Durai et al., 2011; Prakash et al., 2014). The process performance in terms of percentage of chemical oxygen demand (COD) removal of the wastes depends on the degradation of organic pollutants by microbial species and their survival conditions (Bailey et al., 1987; Metcalf et al., 2003). Several substances present in the wastes which inhibit microbial growth kinetics or instabilize process conditions resulted in digester failure. Some of the factors which instabilize process conditions of anaerobic digester are considered in the present investigation. The microbial growth studies have also been carried out under steady-state or transient conditions by Monod model, Contois model, Haldane model, Andrews model and model due to Hill and Barth (Andrews et al., 1971; Hill et al., 1977; Hill 1982). The inhibitory effects on performance of digester by the presence of volatile fatty acids, pH and high loading rates in terms of high influent substrate concentration are also considered. Simulation of the developed model under transient conditions can quantify the extent of process stability which is considered to be of paramount importance in selecting a suitability of a process for specific application. Research on the development of mathematical models for anaerobic digestion process is going on by considering various aspects. Efforts are being made by various researchers again and again for the development of models which fits better and well with the process operations (Angelidaki et al., 1993; Lyberatos et al., 1999; Gavala et al., 2003). The present investigation has been planned to develop transient transport phenomena models across anaerobic digester taking into account most of the pertinent variables that instabilize process conditions with an aim to enhance the stability of digester by considering wall growth factor. Adherence of microorganisms on the digester walls was observed by ZoBell (1943) and Munson (1970). The studies carried out by Munson et al. (1964) showed that the forces by which the microbial species adhere to the walls are electrostatic in nature. Considering wall growth factor Topiwala et al. (1971) developed steady state model equation for microbial growth in a single stage continuous culture. Considering Andrews growth kinetics Azeiteiro et al. (2001) represented the importance of wall growth factor on process performance in terms of COD removal efficiency under high loading rate conditions. The study performed for anaerobic degradation of sewage sludge was restricted to methanogenesis phase. In the present study, a set of dynamic model equations for anaerobic digestion process have been developed based on Haldane growth kinetic model considering both acidogenesis and methanogenesis phases, and wall growth factor associated with methanogenesis step which resists digester failure under the effect of inhibiting agents like volatile fatty acids on methanogens at higher loading rates.
2. Dynamic Model Development
- In the present investigation mathematical model has been developed for continuous complete mixed digester based on the studies of Pavlostathis et al. (1986), Bello-Mendoza et al. (1998) and Azeiteiro et al. (2001). The most important parameter for model building is the data on kinetic coefficients of anaerobic digestion process for simulation purpose. There are numerous sets of data (Andrews 1969; Pavlostathis et al., 1986; Bello-Mendoza et al., 1998) available for sewage sludge treatment with a narrow range of variations. Table 1 depicts representative data on various kinetic coefficients on sewage sludge digestion as reported in the literature, which has been used for simulation in the present investigation. Simulation studies with or without wall growth factor associated with Haldane growth kinetic model has been employed to describe the inhibitory effect of volatile fatty acids on the survival rate of methanogens and on the performance of digester.
|
2.1. Assumptions Made for Development of Model Equations
- The following assumptions were listed as made for development of the present model:Ÿ Continuous complete mix digester Ÿ Microbial growth kinetics of acidogens follow Monod modelŸ Microbial growth kinetics of methanogens described by Haldane modelŸ Hydrolysis and decay coefficient describe by first order kineticsŸ Wall growth factor is limited to methanogenesis phaseŸ Methanogenesis is the rate limiting step in anaerobic digestion process
2.1.1. Monod Growth Kinetic Model
- Based on Monod growth kinetic model the specific and maximum specific growth rate for acidogens are defined as
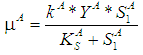 and
and 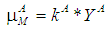 , respectively,and the same for methanogens are given by
, respectively,and the same for methanogens are given by 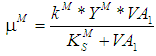 and
and 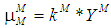 , respectively.
, respectively.2.1.2. Haldane Growth Kinetic Model
- Haldane kinetic model assumes growth limiting characteristics of volatile fatty acids at low concentration and its inhibitory effect on the growth of microorganisms at higher concentration. According to Haldane kinetics, specific growth rate for methanogenic microorganisms is given by
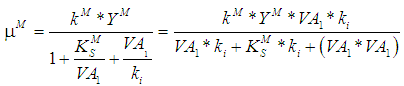 | (1) |
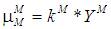 | (2) |
2.2. Model Equations for Continuous Complete Mix Digester
- Using material balance for anaerobic digestion of sewage sludge the model equations as given below are developed. At
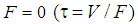 the continuous process model transforms to the model for batch process. The performance of digester has been predicted and compared under two situations with or without wall growth factor. The mass balance equation has been applied across digester to predict the degradation rate of raw substrate (sewage sludge) with respect to time.
the continuous process model transforms to the model for batch process. The performance of digester has been predicted and compared under two situations with or without wall growth factor. The mass balance equation has been applied across digester to predict the degradation rate of raw substrate (sewage sludge) with respect to time.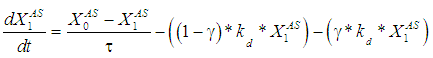 | (3) |
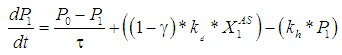 | (4) |
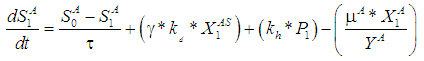 | (5) |
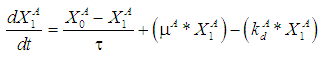 | (6) |
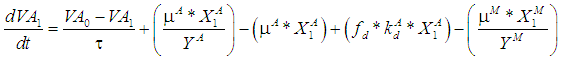 | (7.a) |
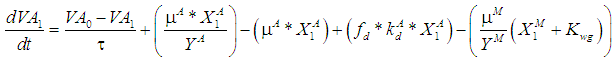 | (7.b) |
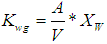 is the wall growth factor.For the estimation of biodegradable fraction of methanogens following equations have been developed.Without wall growth factor:
is the wall growth factor.For the estimation of biodegradable fraction of methanogens following equations have been developed.Without wall growth factor: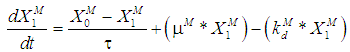 | (8.a) |
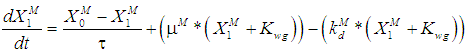 | (8.b) |
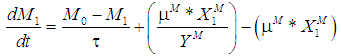 | (9.a) |
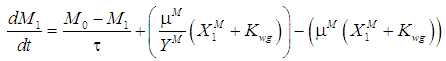 | (9.b) |
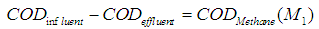 | (10) |
3. Simulation Results and Discussion
- Model Eq. (1) through Eq. (10) were solved simultaneously using Polymath 6.1 and simulations studies were carried out for anaerobic digestion process for sewage sludge under batch and continuous conditions. Influent substrate concentration,
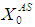 = 10 g/L, particulate substrate concentration,
= 10 g/L, particulate substrate concentration, 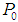 = 0.1 g/L and soluble substrate concentration,
= 0.1 g/L and soluble substrate concentration, 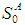 = 5 g/L (Andrews et al., 1971; Bello-Mendoza et al., 1998; Azeiteiro et al., 2001), retention time 10 day under mesophilic (35°C) temperature condition and assuming the value of
= 5 g/L (Andrews et al., 1971; Bello-Mendoza et al., 1998; Azeiteiro et al., 2001), retention time 10 day under mesophilic (35°C) temperature condition and assuming the value of 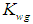 = 0.20 g/L (Azeiteiro et al., 2001).
= 0.20 g/L (Azeiteiro et al., 2001). 3.1. Batch Process
- To study the effect of volatile fatty acid on growth kinetics of methanogens and also on the digester performance, several simulations were performed. Fig. 1 represents the simulation studies performed for batch anaerobic digester at three different values of inhibition constant (for volatile acids),
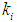 , 0.04 g/L, 0.1 g/L and 0.2 g/L by keeping
, 0.04 g/L, 0.1 g/L and 0.2 g/L by keeping 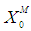 constant at four different values 0.1 g/L, 0.3 g/L, 0.5 g/L and 0.7 g/L. Lower
constant at four different values 0.1 g/L, 0.3 g/L, 0.5 g/L and 0.7 g/L. Lower 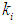 value indicates the higher inhibitory effect on methanogens due to the accumulation of volatile fatty acids, which act as a growth-limiting substrate for methanogens at lower concentration and as inhibiting agent at higher concentration. Fig. 1 represents the effect of volatile fatty acids in terms of
value indicates the higher inhibitory effect on methanogens due to the accumulation of volatile fatty acids, which act as a growth-limiting substrate for methanogens at lower concentration and as inhibiting agent at higher concentration. Fig. 1 represents the effect of volatile fatty acids in terms of 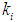 on the survival rate of methanogens. It can be observed from Fig. 1(a) and Fig. 1(b) that when the digester is operated at lower values of
on the survival rate of methanogens. It can be observed from Fig. 1(a) and Fig. 1(b) that when the digester is operated at lower values of 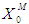 (0.1 g/L and 0.3 g/L) survival rate of methanogens were inhibited even at higher values of
(0.1 g/L and 0.3 g/L) survival rate of methanogens were inhibited even at higher values of 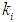 = 0.2 g/L (less inhibitory effect). The maximum survival rate of methanogens at higher value of
= 0.2 g/L (less inhibitory effect). The maximum survival rate of methanogens at higher value of 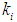 = 0.2 g/L was found to be 0.119 g/L and 0.407 g/L for initial concentration,
= 0.2 g/L was found to be 0.119 g/L and 0.407 g/L for initial concentration, 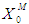 = 0.1 g/L (Fig. 1(a)) and 0.3 g/L (Fig. 1(b)), respectively. Lower
= 0.1 g/L (Fig. 1(a)) and 0.3 g/L (Fig. 1(b)), respectively. Lower 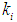 values of 0.1 g/L and 0.04 g/L resulted in process failure due to high inhibitory effect of volatile fatty acids on methanogens. As shown in Fig. 1(c) and Fig. 1(d), when the digester was operated at higher values of
values of 0.1 g/L and 0.04 g/L resulted in process failure due to high inhibitory effect of volatile fatty acids on methanogens. As shown in Fig. 1(c) and Fig. 1(d), when the digester was operated at higher values of 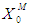 = 0.5 g/L and 0.7 g/L the survival rate of methanogens was increased from 0.5 g/L to 0.6 g/L and 0.7 g/L to 1.04 g/L, respectively, at lower value of
= 0.5 g/L and 0.7 g/L the survival rate of methanogens was increased from 0.5 g/L to 0.6 g/L and 0.7 g/L to 1.04 g/L, respectively, at lower value of 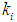 = 0.1 g/L. Maximum survival rate was observed at higher values of
= 0.1 g/L. Maximum survival rate was observed at higher values of 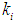 = 0.2 g/L (Fig. 1). From the batch studies it was observed that during initial phase the concentration of substrate is much higher resulted into higher production rate of volatile fatty acids (lower value of
= 0.2 g/L (Fig. 1). From the batch studies it was observed that during initial phase the concentration of substrate is much higher resulted into higher production rate of volatile fatty acids (lower value of 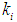 ) which effects the growth kinetics of methanogens. Operating digester at high concentration of volatile fatty acids causes poor process performance.Therefore, the digester should be operated at higher values of
) which effects the growth kinetics of methanogens. Operating digester at high concentration of volatile fatty acids causes poor process performance.Therefore, the digester should be operated at higher values of 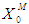 and
and 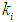 to reduce the time required for start-up of the digester. This is in agreement with the observations of other researchers (Andrews et al., 1971; Azeiteiro et al., 2001).
to reduce the time required for start-up of the digester. This is in agreement with the observations of other researchers (Andrews et al., 1971; Azeiteiro et al., 2001).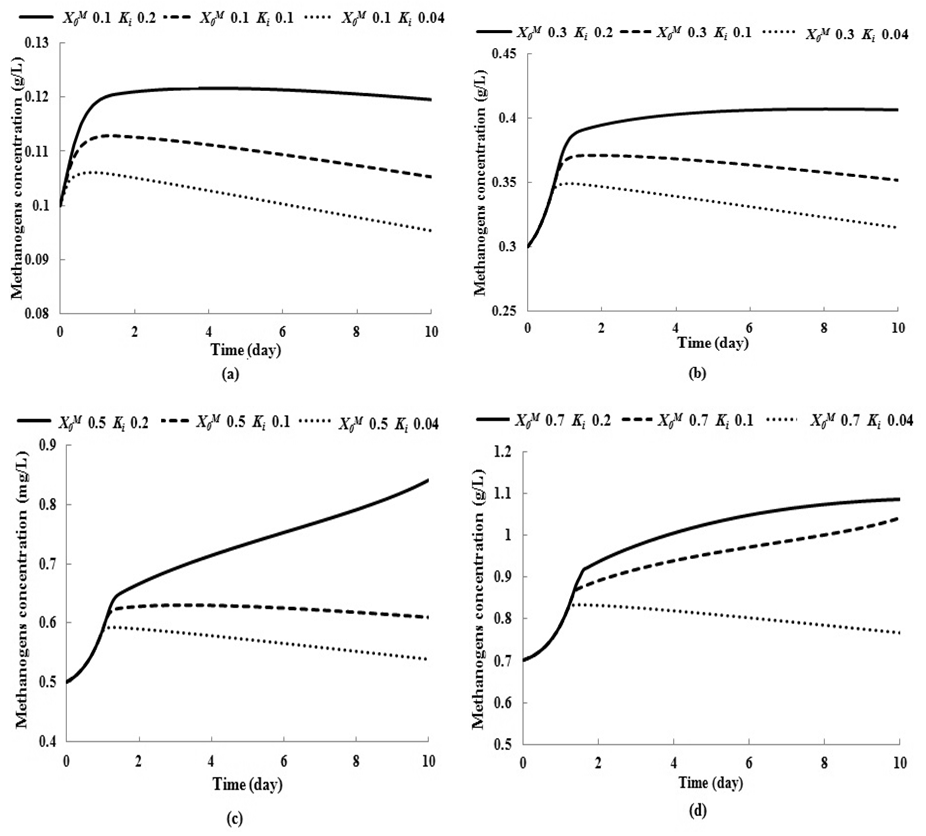 | Figure 1. Simulation studies for batch anaerobic digester with varying inhibition constant |
3.2. Continuous Process
- Simulations for the continuous process have been carried out to predict the effect of volatile fatty acids and initial microbial concentration,
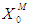 , on digester performance under transient conditions by varying the value of
, on digester performance under transient conditions by varying the value of 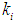 and
and 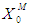 , respectively. The results of simulation of continuous processes have been shown in Fig. 2 and Fig. 3. In Fig. 2 and Fig. 3, time (day) has been plotted against both methanogens concentration (g/L) and CODMethane (g COD/L). COD removal efficiency can be predicted by the formula given below. Fig. 2(a) and Fig. 2(b) describe the effect of
, respectively. The results of simulation of continuous processes have been shown in Fig. 2 and Fig. 3. In Fig. 2 and Fig. 3, time (day) has been plotted against both methanogens concentration (g/L) and CODMethane (g COD/L). COD removal efficiency can be predicted by the formula given below. Fig. 2(a) and Fig. 2(b) describe the effect of 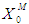 on the COD removal and survival rate of methanogens under constant value of inhibitory conditions (
on the COD removal and survival rate of methanogens under constant value of inhibitory conditions (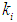 =0.2 g/L). COD removal efficiency could be evaluated by using the following expression due to Bello-Mendoza et al. (1998):
=0.2 g/L). COD removal efficiency could be evaluated by using the following expression due to Bello-Mendoza et al. (1998):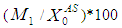 | (11) |
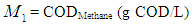
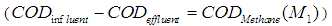 The maximum COD removal efficiency of anaerobic digestion of sewage sludge in the present investigation was found of the order of 55.2% at higher values of
The maximum COD removal efficiency of anaerobic digestion of sewage sludge in the present investigation was found of the order of 55.2% at higher values of 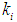 = 0.2 g/L and
= 0.2 g/L and 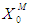 = 0.7 g/L as shown in Fig. 2(b). It was also observed from Fig. 2(a) and Fig. 2(b) that even under low inhibitory effect (high
= 0.7 g/L as shown in Fig. 2(b). It was also observed from Fig. 2(a) and Fig. 2(b) that even under low inhibitory effect (high 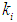 value), lower values of
value), lower values of 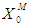 (0.1 g/L and 0.3 g/L) result in lower microbial survival rates indicating poor COD removal. Fig. 3(a) and Fig. 3(b) describe the effect different values of
(0.1 g/L and 0.3 g/L) result in lower microbial survival rates indicating poor COD removal. Fig. 3(a) and Fig. 3(b) describe the effect different values of 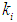 on constant initial microbial concentration,
on constant initial microbial concentration, 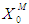 = 0.7 g/L. At lower
= 0.7 g/L. At lower 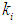 values of 0.04 g/L and 0.02 g/L higher inhibitory effect of volatile fatty acids on methanogens resulted in decrease in
values of 0.04 g/L and 0.02 g/L higher inhibitory effect of volatile fatty acids on methanogens resulted in decrease in 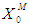 value from 0.7 to 0.283 g/L and 0.266 g/L (Fig. 3(a), respectively. At higher
value from 0.7 to 0.283 g/L and 0.266 g/L (Fig. 3(a), respectively. At higher 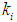 values less inhibition resulted in higher values of both COD removal efficiency and
values less inhibition resulted in higher values of both COD removal efficiency and 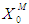 (Fig. 3(a) and Fig. 3(b). Therefore, to improve the performance higher values of both
(Fig. 3(a) and Fig. 3(b). Therefore, to improve the performance higher values of both 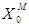 and
and 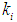 should be maintained when the digester is operated at transient conditions.
should be maintained when the digester is operated at transient conditions. 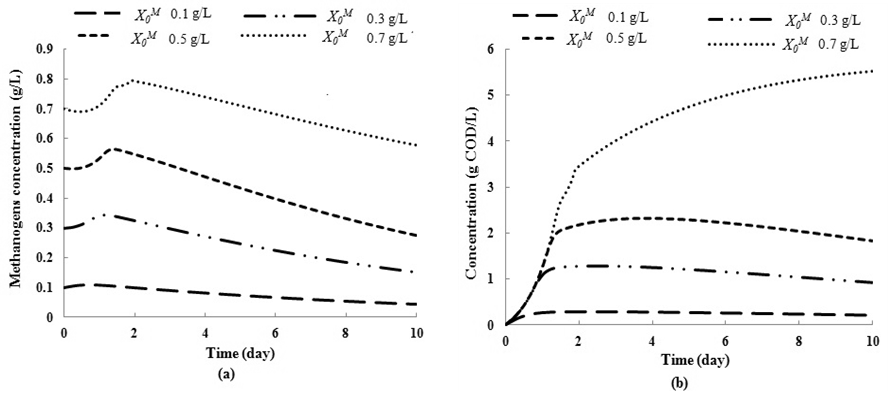 | Figure 2. Simulation studies for effect on (a) methanogenic growth kinetic and (b) COD removal for continuous anaerobic digester with varying initial methanogens concentration ( Ki = 0.2 g/L) |
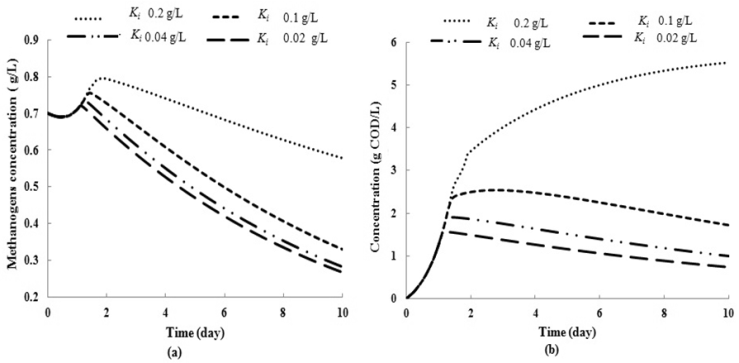 | Figure 3. Simulation studies for effect on (a) methanogenic growth kinetic and (b) COD removal for continuous anaerobic digester with varying inhibition constant (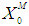 = 0.7 g/L) = 0.7 g/L) |
3.2.1. Effect of Dilution on Digester Performance for Sewage Sludge at High Loading Rates
- This section presents simulation studies performed under transient conditions with variation in both the loading rate (by increasing the influent substrate concentration) with respect to dilution rate (by increasing retention time). In Fig. 4, time (day) has been plotted against CODMethane (g COD/L).Fig. 4 represents the simulation results under the effect of increasing loading rate at constant retention time at
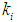 = 0.2 g/L and
= 0.2 g/L and 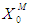 = 0.7 g/L. Increase in
= 0.7 g/L. Increase in 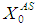 value from 10 g/L to 25 g/L (Fig. 4(a)) and from 10 g/L to 40 g/L (Fig. 4(b)) at same retention time of 15 day instabilize digester conditions and resulted in decreases in COD removal from 64% to 13.8% and 5.5%, respectively. Operating digester at higher loading rate at shorter retention time leads to the formation of volatile fatty acids at a rate higher than its rate of utilization which results in reduction of survival rate of methanogens. However, stability of digester at higher loading rates can be enhanced by simultaneously increase in retention time or diluting the reactor contents. Fig. 4(c) through Fig. 4(f) also represents the simulations carried out for gradual increase in loading rates. The retention time and substrate concentration
value from 10 g/L to 25 g/L (Fig. 4(a)) and from 10 g/L to 40 g/L (Fig. 4(b)) at same retention time of 15 day instabilize digester conditions and resulted in decreases in COD removal from 64% to 13.8% and 5.5%, respectively. Operating digester at higher loading rate at shorter retention time leads to the formation of volatile fatty acids at a rate higher than its rate of utilization which results in reduction of survival rate of methanogens. However, stability of digester at higher loading rates can be enhanced by simultaneously increase in retention time or diluting the reactor contents. Fig. 4(c) through Fig. 4(f) also represents the simulations carried out for gradual increase in loading rates. The retention time and substrate concentration 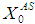 were varied from 15 day to 30 day and 20 g/L to 50 g/L, respectively. Increasing
were varied from 15 day to 30 day and 20 g/L to 50 g/L, respectively. Increasing 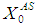 value from 10 g/L to 20 g/L at retention time of 15 day resulted in decrease in COD removal from 64% to 48% (Fig. 4(c), whereas increase in
value from 10 g/L to 20 g/L at retention time of 15 day resulted in decrease in COD removal from 64% to 48% (Fig. 4(c), whereas increase in 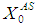 value from 20 g/L to 25 g/L at the same retention time of 15 day resulted in decreases of COD removal efficiency from 48% to 13.8%. Increase in
value from 20 g/L to 25 g/L at the same retention time of 15 day resulted in decreases of COD removal efficiency from 48% to 13.8%. Increase in 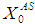 value from 30 g/L to 35 g/L at the same retention time of 20 day resulted in decrease in COD removal from 46.8% to 8.7% as shown in Fig. 4(d). Operating digester at higher loading rate at same retention time results in build up of volatile fatty acids and its inhibitory effects on methanogens. As shown in Fig. 4(e), when retention time was increased from 20 day to 25 day at constant loading rate of 35 g/L COD removal improved from 8.7% to 48.6%. This is due to dilution of reactor contents.Again increase in
value from 30 g/L to 35 g/L at the same retention time of 20 day resulted in decrease in COD removal from 46.8% to 8.7% as shown in Fig. 4(d). Operating digester at higher loading rate at same retention time results in build up of volatile fatty acids and its inhibitory effects on methanogens. As shown in Fig. 4(e), when retention time was increased from 20 day to 25 day at constant loading rate of 35 g/L COD removal improved from 8.7% to 48.6%. This is due to dilution of reactor contents.Again increase in 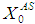 value from 40 g/L to 50 g/L at the same retention time of 30 day resulted in decrease in COD removal efficiency from 49.8% to 6.6% due to build up of volatile fatty acids as shown in Fig. 4(f).
value from 40 g/L to 50 g/L at the same retention time of 30 day resulted in decrease in COD removal efficiency from 49.8% to 6.6% due to build up of volatile fatty acids as shown in Fig. 4(f). 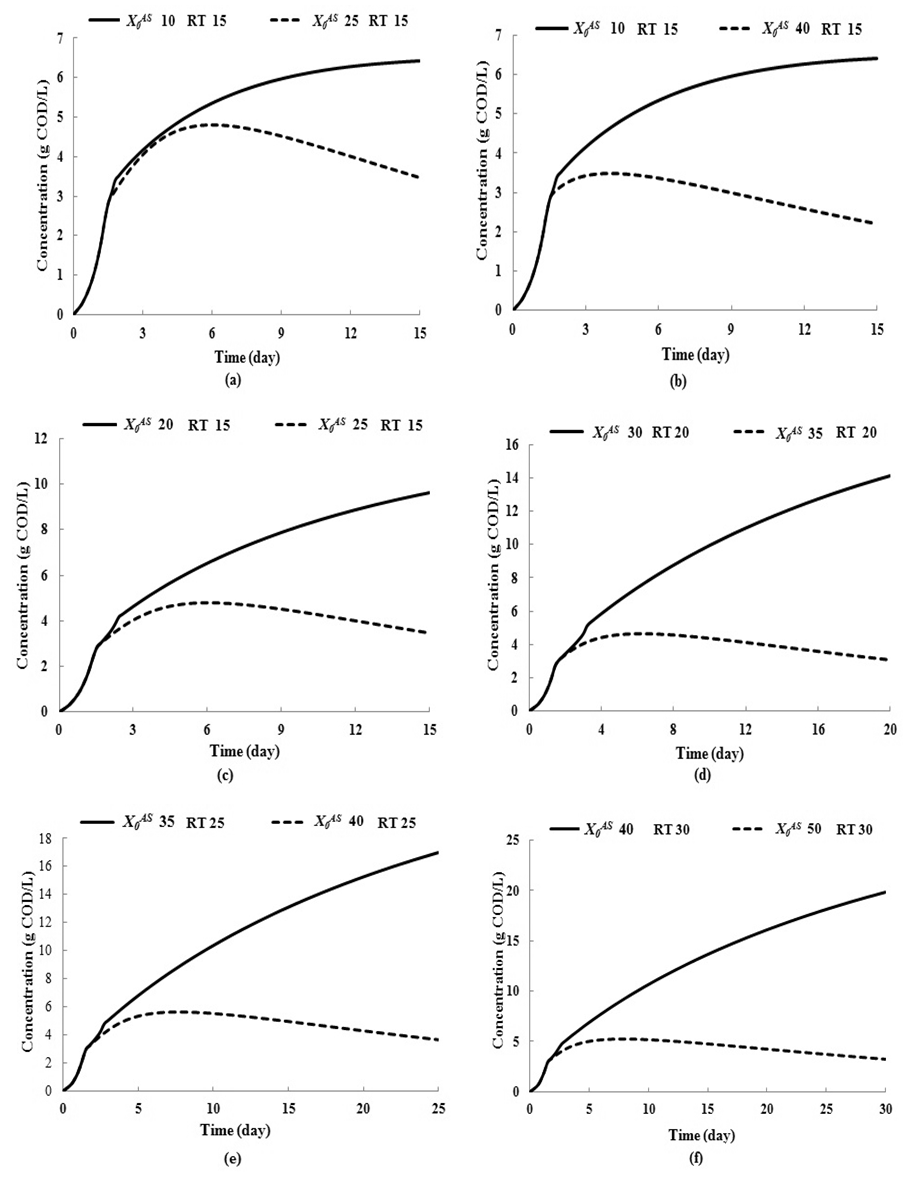 | Figure 4. Simulation studies for continuous anaerobic digestion process for increase in loading rates (ki 0.2 g/L and 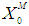 0.7 g/L) and effect of dilution on process performance 0.7 g/L) and effect of dilution on process performance |
3.3. Simulation Studies at Higher Loading Rates with or without Wall Growth Factor
- Adherence of microorganisms on the walls of the digester and the importance of wall growth factor has been well defined in literature (Zo-Bello 1943; Larsen et al., 1964; Munson et al., 1964; Munson 1970; Topiwala et al., 1971). The microorganisms adhering to the walls of the digester reduce the concentration of volatile fatty acids (by degradation of substrate), thus reduce its inhibitory effect to methanogens and also resist digester failure under transient conditions. In the present section simulation of model equations (Fig. 5) associated with wall growth factor has been carried out considering Haldane growth kinetic model under transient conditions. In Fig. 5, time (day) has been plotted against CODMethane (g COD/L).
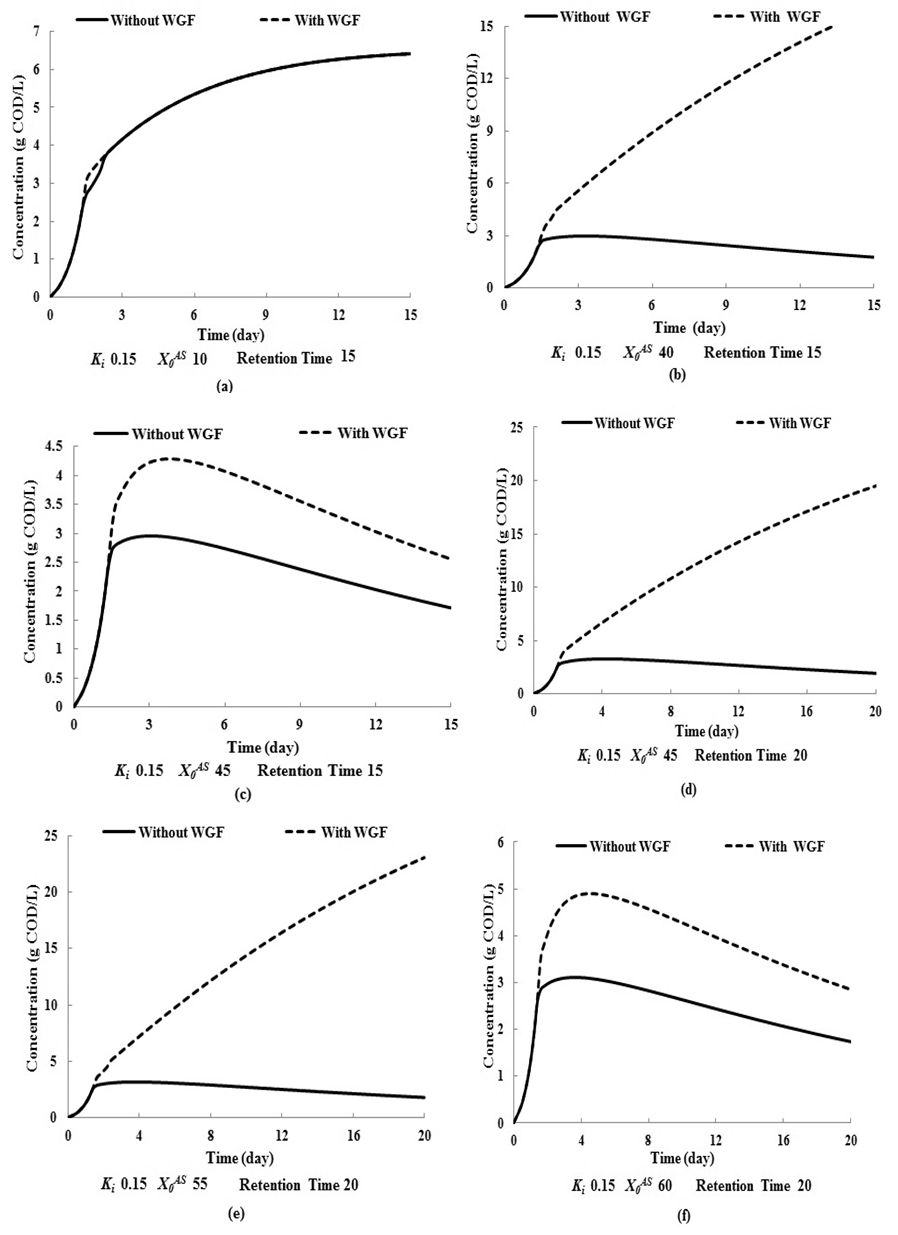 | Figure 5. COD removal efficiency of anaerobic digester with or without wall growth factor |
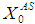 value increases from 10 g/L to 50 g/L at same retention time of 15 day (
value increases from 10 g/L to 50 g/L at same retention time of 15 day (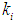 = 0.15 g/L and
= 0.15 g/L and 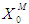 = 0.7 g/L). As shown in Fig. 5(a) at low value of
= 0.7 g/L). As shown in Fig. 5(a) at low value of 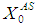 =10 g/L, no variation in COD removal efficiency indicates that there is no significant inhibitory effect of volatile fatty acids on the methanogens prevails at this retention time. The variation of
=10 g/L, no variation in COD removal efficiency indicates that there is no significant inhibitory effect of volatile fatty acids on the methanogens prevails at this retention time. The variation of 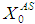 from 10 g/L to 40 g/L at the same retention time of 15 day (Fig. 5(b)), results in higher COD removal rates when compared with the simulation results of digester operated without considering wall growth factor. This is in agreement with the results obtained by Azeiteiro et al. (2001) under high loading rate conditions. Table 2 describes the comparison of the results observed when digester is operated with or without wall growth factor. If the digester is operated at higher
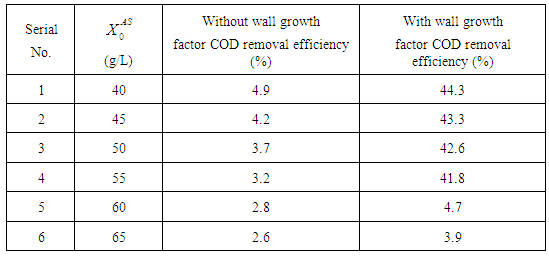
from 10 g/L to 40 g/L at the same retention time of 15 day (Fig. 5(b)), results in higher COD removal rates when compared with the simulation results of digester operated without considering wall growth factor. This is in agreement with the results obtained by Azeiteiro et al. (2001) under high loading rate conditions. Table 2 describes the comparison of the results observed when digester is operated with or without wall growth factor. If the digester is operated at higher 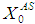 (between 40 g/L to 50 g/L) build up of volatile fatty acids cause inhibition to methanogens resulting in sharp decrease in COD removal efficiency at the same retention time of 15 day as observed in the case associated with wall growth factor (Fig. 5(c)). Operating digester at higher loading rate requires dilution in both the cases for optimizing process performance.
(between 40 g/L to 50 g/L) build up of volatile fatty acids cause inhibition to methanogens resulting in sharp decrease in COD removal efficiency at the same retention time of 15 day as observed in the case associated with wall growth factor (Fig. 5(c)). Operating digester at higher loading rate requires dilution in both the cases for optimizing process performance.
|
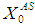 value from 40 g/L to 45 g/L at retention time of 20 day (Fig. 5(d)). However, further increase in
value from 40 g/L to 45 g/L at retention time of 20 day (Fig. 5(d)). However, further increase in 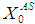 value from 55 g/L (Fig. 5(e)) to 60 g/L (Fig. 5(f)) resulted in instability of the digester conditions as COD removal efficiency decreased from 41.8% to 4.7%.
value from 55 g/L (Fig. 5(e)) to 60 g/L (Fig. 5(f)) resulted in instability of the digester conditions as COD removal efficiency decreased from 41.8% to 4.7%.
|
4. Conclusions
- The developed dynamic model helps in evaluating the stable conditions for proper digester working and helpful in controlling the process due to inhibitory effects of total volatile fatty acids on methanogens. Poor efficiency and decrease in survival rate of microorganisms at lower concentration (
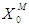 ) of the range between 0.1 g/L - 0.3 g/L was observed even at higher
) of the range between 0.1 g/L - 0.3 g/L was observed even at higher 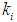 values in both batch and continuous processes. In order to avoid these situations, digester should be operated at higher values of
values in both batch and continuous processes. In order to avoid these situations, digester should be operated at higher values of 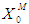 and
and 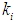 . During the start-up of the digester, the value of the microbial concentration should be high. Higher
. During the start-up of the digester, the value of the microbial concentration should be high. Higher 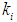 (0.2 g/L) and
(0.2 g/L) and 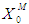 (0.7 g/L) values resulted in higher COD removal efficiency (55.2%) for the continuous process at a retention time of 10 day and at
(0.7 g/L) values resulted in higher COD removal efficiency (55.2%) for the continuous process at a retention time of 10 day and at 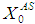 of 10 g/L. COD removal efficiency decreases even at the same
of 10 g/L. COD removal efficiency decreases even at the same 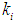 (0.2 g/L) but with lower
(0.2 g/L) but with lower 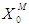 value (0.5 g/L, 0.3 g/L and 0.1 g/L). This further indicates that proper acclimatization of microorganisms and constant process conditions are needed for better performance of digester.Consideration of wall growth factor in the model enhances the performance of digester as COD removal efficiency increases from 12% to 48% at the same
value (0.5 g/L, 0.3 g/L and 0.1 g/L). This further indicates that proper acclimatization of microorganisms and constant process conditions are needed for better performance of digester.Consideration of wall growth factor in the model enhances the performance of digester as COD removal efficiency increases from 12% to 48% at the same 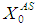 value of 20 g/L and retention time of 15 day. Dilution factor plays an important role under the inhibitory effect of volatile fatty acids in both situations, with or without wall growth factor. A step increase in substrate concentration
value of 20 g/L and retention time of 15 day. Dilution factor plays an important role under the inhibitory effect of volatile fatty acids in both situations, with or without wall growth factor. A step increase in substrate concentration 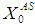 from 40 g/L to 45 g/L at the retention time of 15 day resulted in poor COD removal efficiency of 6.6%, while increasing retention time to 20 day increased the same to 43.3% under similar conditions. However, operating digester at higher retention time results in increase in the cost of process operations. Therefore, wall growth factor should always be considered for dynamic modelling and simulation of anaerobic digestion processes.
from 40 g/L to 45 g/L at the retention time of 15 day resulted in poor COD removal efficiency of 6.6%, while increasing retention time to 20 day increased the same to 43.3% under similar conditions. However, operating digester at higher retention time results in increase in the cost of process operations. Therefore, wall growth factor should always be considered for dynamic modelling and simulation of anaerobic digestion processes.ACKNOWLEDGEMENTS
- This work has been supported by Ministry of Human Resources and Development (MHRD), Government of India, India.
Nomenclature

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML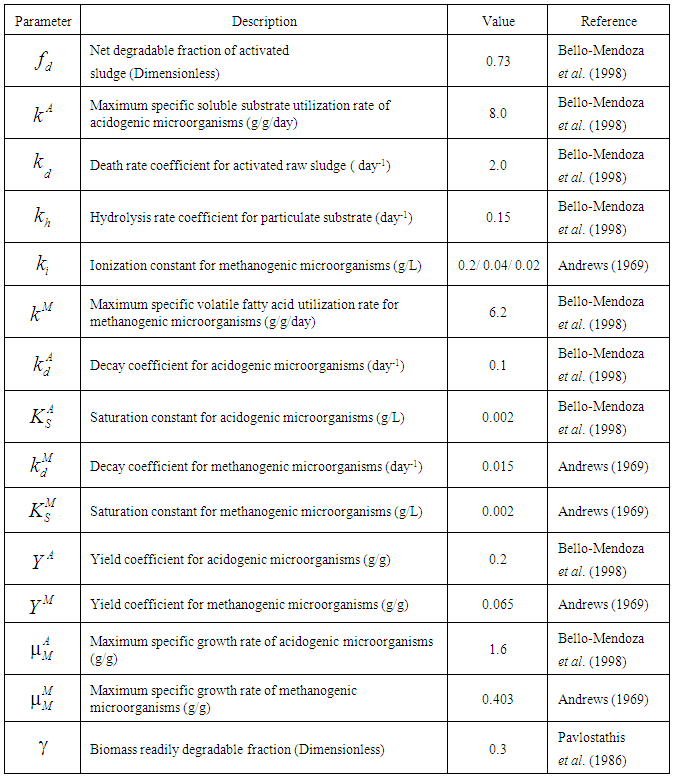
 = 0.7 g/L)
= 0.7 g/L) 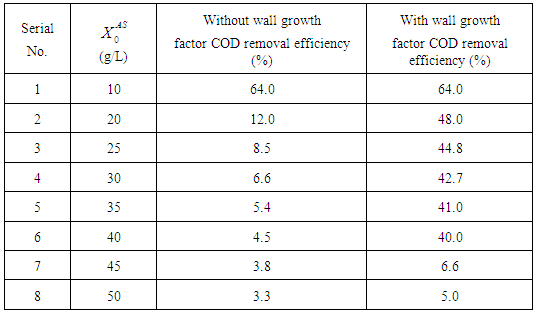
 = 0.7 g/L)
= 0.7 g/L)