-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2016; 6(3): 88-98
doi:10.5923/j.ajee.20160603.02

Water Pollution: Source & Treatment
Sulaiman A. Alrumman1, Attalla F. El-kott2, Sherif M. A. S. Keshk3
1King Khalid University, Faculty of Science, Biological Department, Abha, Saudi Arabia
2Zoology Dept. Faculty of Science, Damanhour University, Damanhour, Egypt
3Chemistry Department, Faculty of Science, King Khalid University, Abha, Saudi Arabia
Correspondence to: Sherif M. A. S. Keshk, Chemistry Department, Faculty of Science, King Khalid University, Abha, Saudi Arabia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Water covers about 70% of the Earth's surface whereas 0.002% of the water is available for human consumption. Contaminated water is the main source of infectious diseases (e.g. Amoebiasis and Malaria, Cholera, Dysentery, Paratyphoid Fever, Typhoid, Jaundice). The WHO reports that one sixth of the world’s population (1.1 billion people) does not have access to safe water. Water pollutions that come from industry, agriculture or households, returns negatively back to the environment. Chemical wastes (e.g. Arsenic, Fluorides, Lead, Nitrates, Pesticides, Petro-chemicals) in the water have negative effect on living organism in water and subsequently on our health. The effects of water pollution are varied and depend on chemicals kinds that dumped and their locations (urban areas are highly polluted). Pollutants such as lead and cadmium are consumed by tiny animals. Later, the food chain continues to be disrupted at all higher levels. Several countries sought to regulate the discharges of pollutants in the water to minimize pollution and contamination through various treatments. In this review, we are going to explain the main source of water pollution to promote sustainable use of water. Moreover, ensuring the highest protection of water from all hazardous chemicals.
Keywords: Source of pollution, Water pollutant, Hazardous chemicals, Infectious diseases
Cite this paper: Sulaiman A. Alrumman, Attalla F. El-kott, Sherif M. A. S. Keshk, Water Pollution: Source & Treatment, American Journal of Environmental Engineering, Vol. 6 No. 3, 2016, pp. 88-98. doi: 10.5923/j.ajee.20160603.02.
Article Outline
1. Introduction
- Water pollution occurs when undesirable effluents disperse in a water system and so water quality change. Water pollution divided into three main sources, natural Sources: include thermal and acid effluents from volcanic areas and are not common on the earth, domestic sources that are primarily sewage and laundry wastes and generated in houses, apartments, and other dwellings. In rural and some suburban areas, domestic wastes are handled at the individual residence and enter the environment through the soil either in partially treated or untreated fashion. In urban areas, domestic wastes are collocated in sewage pipes and transmitted to control location either for treatment or discharge into a watercourse without treatment (This considered as the major potential source of water pollution). Urban sewage since they handled by established government agencies, they can usually be effectively controlled (Boyd and Tucker, 2012). Industrial wastes vary from industry to industry and from location to location. Some industries generate wastes high in organic matter, and these wastes can usually handled by methods similar to those used for domestic wastes, such industries include dairy and food-processing plants, meat-packing houses. Other industries, however, generate wastes that are low in organic matter but high in toxic chemicals such as metals, acids or alkalis. These include chemical plants, mining facilities, and textile mills (Nesaratnam, 2014, Williams et al., 2015).
2. Type of Pollutants
- There are many types of pollutants such as Oxygen demanding wastes; disease-causing agents; plant nutrients; organic chemicals; inorganic chemicals; sediments; radioactive substances and heat. In most situations, the waste treated is a mixture of the preceding types of pollutants, thus greatly complicating treatment and control procedures (Nesaratnam, 2014).Algae and Water PollutionA serious problem in many lakes and reservoirs used as sources of water is the growth of algae. Algae are undesirable because they cause bad odors and flavors in water and may produce toxic materials of potential danger to human. Algal growth favored by warm water temperatures, high sunlight, adequate a source of nutrients especially nitrates phosphates and carbon dioxide. Therefore, algal growth is most common in summer and is rare in winter. Occasionally, in late summer and early fall, algal growth may be so heavy that water resembles pea soup. This condition called an algal bloom (Palmer, 1980; Laliberte et al., 1994; Kamarudin et al., 2015). When algae float to the surface and drift into backwaters where they become concentrated. Bacteria attack and decompose them causing a reduction in oxygen, which in turn leads to the death of fish and other animals and the development of foul and putrefying odors (Glibert, 2014). Reservoirs for domestic water supplies are often good habitats for algal growth because they are relatively shallow and receive large amounts of algal nutrients from the watershed. In addition to the odor and flavor problems that may develop, heavy algal growth in reservoir makes filtration and disinfection of the water difficult, thus markedly increasing the coast of water purification. Algal growth in reservoirs controlled by the application of copper sulfate. In many water supplies, copper sulfate is applied routinely from two to four weeks. The exact amount of copper sulfate required depends on the alkalinity or acidity of the reservoir. Copper sulfate use in reservoirs must be carefully controlled because copper sulfate is toxic to fish and in high doses to humans (Paerl et al., 2001). Algal growth in swimming pools causes an unsightly slime on the walls of the pool and reduces water clarity. The best method of control is by chlorination, but if algal growth is heavy, copper sulfate treatment may be used (Hamilton, 1989; Spector, 2001; Hasskerl et al., 2015). EutrophicationSewage not only contributes organic matter to the growth of bacteria but nutrients for the growth of algae. The enrichment of water courses with algal nutrients is called eutrophication and is a serious economic problem because algal growth adds organic matter back into the water, thus increasing the BOD that causing a deterioration of water quality. Nitrate and phosphate are especially important in water pollution because they are effective nutrient sources for algae (organic nitrogen converted to ammonia, then ammonia is oxidized to nitrate, and organic phosphorus converted to inorganic phosphate) (Boyd and Tucker, 2012). Since conventional sewage treatment does not eliminate algal nutrients, eutrophication can be prevented if advanced sewage treatment methods used. The elimination of nitrate is more complicated, as this anion is not perceptible by any of the agents used in advanced treatment systems (Craggs et al., 2012). One method for elimination of nitrate is de-nitrification using bacteria (Cheikh et al., 2013).Biochemical Oxygen Demand (BOD)Biochemical Oxygen Demand one of the most important factor that depleted the dissolved oxygen in the water. Because, water contains organic matter and bacteria that oxidize organic matter, in the process consuming oxygen (Baldry et al., 1991). The decrease in oxygen content has several important consequences: i. it makes the watercourse partially or completely anaerobic, and this lead to the development of odors, flavors, and toxic materials in the water. ii. When the water becomes anaerobic, many animals such as fish die, and their remains putrefy and add further foul odors and organic matter to the water. iii. Even if odors do not develop, water depleted of oxygen has a flat taste. iv. Decomposition of organic materials takes place much more slowly in the absence of oxygen; the purification process in the water course are therefore slow, and a thick, unsightly, organic-rich sediment may accumulate on the bottom of the watercourse. v. Certain undesirable animals, such as the red bloodworms (chironomid larvae), develop to very large numbers in waters depleted of oxygen. The consumption of oxygen by bacteria is called the biochemical oxygen demand, usually abbreviated BOD, the extent of oxygen consumption determined by the amount of oxidizable organic matter present in the water. The BOD commonly used as a measure of organic pollution degree in waters. The BOD evaluated by aerating the water sample well, then placing it in a sealed bottle, incubating for the standard period (usually five days at 20°C), and then determining the residual oxygen at the end of incubation. During the 5-day incubation period, microorganisms present in the water grow, oxidize the organic matter and consume oxygen (Fennel, 2014). The amount of oxygen consumed is roughly proportional to the amount of biodegradable organic matter. Chemical Oxygen Demand (COD)Chemical Oxygen Demand is another evaluation that used to measure the level of water contamination by organic matter. In this evaluation, the organic matter is oxidized via oxidizing agent (potassium dichromate). The COD is usually higher than the BOD because some organic materials in the water that are resistant to microbial oxidation and hence not involved in BOD will be easily chemically oxidized (Chen et al., 2014, Gattrell, 2014). Self-Purification of WaterWhen sewage added to a river or other watercourse, pollution occurs. Pollution followed by purification, the process in which the quality of the water returned toward normal. When purification occurs without human intervention, it is called self-purification and occurs as a result of microbiological, chemical, and physical changes. Microbiological changes include the death of many intestinal microorganisms present in the sewage and growth of normal aquatic microorganisms able to oxidize organic matter entering the system. Chemical changes include oxidation of organic matter, the release of phosphate, nitrate and re-oxygenation of the water by the \ oxygen solution from the air. The most important physical changes involve sedimentation, in which particulate matter settles out of the water onto the bottom of the watercourse (Ostroumov, 2014, Drewniak et al., 2015). Microbial Indicators of Sewage PollutionMicrobial indicators provide an excellent means of monitoring natural water for sewage pollution; because they easily detected. Any of the organisms used in evaluating drinking water for microbial purity can used as indicators of sewage pollution. The most frequently two indicators used are the coliform group and the subgroup of the coliforms (fecal coliforms). In general, any gram-negative, rod-shaped, facultative anaerobic bacterium is called coliform (Escherichia coli is typical coliform). The fecal coliform group defined as containing those coliforms can grow at an elevated temperature of 44.5°C. This elevated temperature is suitable for organisms associated with the intestinal tract and eliminates many of the no intestinal coliforms able to grow at the standard temperature of 35°C. The fecal coliforms are thus better indicators of recent sewage pollution (Kapoor et al., 2015, Sivaraja and Nagarajan, 2014). Furthermore, measuring enzymatic activities can provide information about the function and structure of microbial communities in contaminated media. These measures could use as rapid and cost-effective means for evaluating and monitoring remediation of contaminated media (Alrumman et al., 2015).
3. Water Treatment Methods
- We will present only a brief, general account of typical four series steps. Sedimentation If the water source is highly turbid, the raw water is pumped into lagoons and allowed to stand for several hours. Silt and other materials sink to the bottom, and certain flocculating chemicals are added to contaminated water to precipitate and absorb finer particles (García et al., 2014). Filtration The water pumped from the settling areas into tanks that equipped with sand filters. The water is cleared from the most remaining impurities, including numerous bacteria and other microorganisms (Cui and Choo, 2014, Katsoyiannis et al., 2015). Aeration If the water contains large quantities of organic material, it may be sprayed into the air or allowed to flow over a series of shallow waterfalls to increase the availability of oxygen to microorganisms capable of oxidizing organic compounds (Hadad and Ghaderi, 2015, Shao et al., 2015). Chlorination Either chlorine gas or certain chlorine compounds are added to water to complete the purification process. Extremely small amounts of chlorine, about 0.3 parts per million, are usually adequate to kill almost all the microorganisms (certainly all the pathogens) remaining in the water. At the same time, chlorine neutralizes many odors and tastes in the water. The final product is then ready to be distributed to the public (Salgado et al., 2015, Speich et al., 2015). As we have indicated, there are many variations of these procedures. At one extreme water that are so pure that they require only chlorination or no treatment whatsoever. At the other extreme water that contain so many impurities that they must receive more elaborate treatment. For example, some waters contain large amounts of calcium, magnesium, or iron salts that must be removed by special chemical procedures to "soften" the water. Regardless of the initial quality of the water, fluorides may be added to reduce the incidence of dental caries (Pennel et al., 2015).
4. Sewage
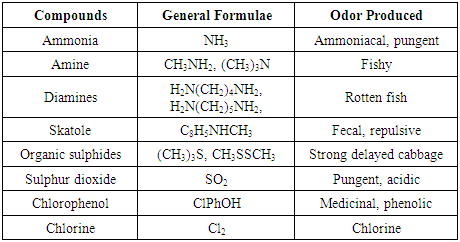
- Sewage is a mixture of natural organic and inorganic materials with a small ratio of man-made substances. The main source of sewage polluted is human excreta with food preparation from contributions and surface drainage. The physical and chemical nature of water wastes can be further complicated by industrial wastes that are composed of strong spent liquors from main industrials processes. Domestic wastewater comes mainly from the residence, commercial buildings, and institutions such as schools and hospitals, whereas, industrial wastewater comes from manufacturing plants. Inevitably, large towns and cities have a mixture of domestic and industrial wastewater, which is commonly referred to as municipal wastewater (de Mora and Harrison, 2013). The other wastewater that rich in organic materials and readily biodegraded are the agro industrial wastes, these wastes varied according to agricultural practice, manufacturing processes and from intensive animal rearing, silage production, food processing and the dairy industry (VikranthPridhvi and Musalaiah, 2015). Physical PropertiesThe vast majority of the large solids, such as faces and paper have broken up into very small particles and made turbidity with visible particles of organic material. The water color become gray and change to yellow-brown, according to the time day. However, if all the oxygen has been consumed during transit in the sewer then the wastewater becomes anaerobic or septic. Thus water become much darker color and in extreme eases turns black (Sell, 1992, Olariu, 2015). So, the contaminated water problems are related to odors, corrosiveness (pH), and turbidity. OdorsPhormidium, Actinomycetes and Streptomyces. Wastewater (becomes anaerobic) has a musty smell that is not at all offensive. Microorganisms that produce such odor are Cyanobacteria, Oscillatoria, Moreover, certain industrial wastes have distinctive odors that caused by gasses involved from decomposition of various fractions of the organic matter. The rotten eggs is the commonest odor that caused by hydrogen sulfide produced by anaerobic bacteria (reduction of sulfate to sulfide). On the other hand, volatile fatty acids odor produced during food processing treatment and storage (table 1). Where, carbohydrates wastewater undergoes partial anaerobic breakdown within the process and subsequently on storage in lagoons. For example, the volatile acids odor concentrations during sugar beet treatment are 24.3 ppm for acetic acid, 20.0 ppm for propionic acid, 0.05 ppm for iso-butyric acid and 0.24 ppm for butyric acid, 0.7 ppm for iso-valeric acid and 3.0 ppm for valeric acid. Other odors associated with sugar beet processing are the fishy odor come from trimethylamine and rotting cabbage produced from organic sulphides and the thiol compounds (table 1) (Dague, 1972, Muramoto et al., 1995, Loan et al., 2014). There are other odorous associated with chlorine and phenolic wastes as in the following table 1:
|
5. Sewage Treatment
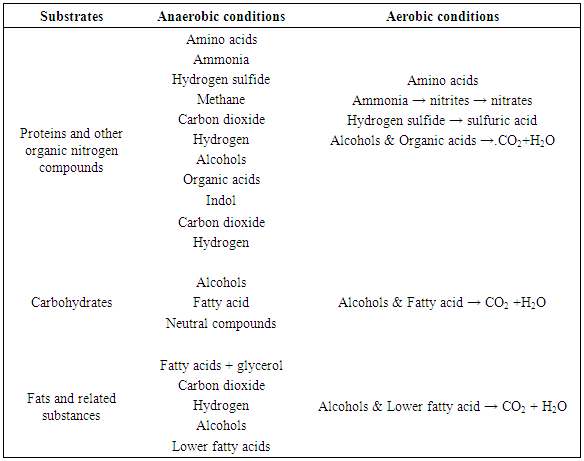
- Sewage treatment consists of a series of processes in which undesirable materials in the water are removed or rendered harmless. BOD destroyed, silt clay and other debris removed, pathogenic microorganisms killed, and the total number of microorganisms reduced. There are many designs for sewage-treatment systems; the best design to use for a specific system depends on local factors. Both biological and nonbiological treatments are used. The biological treatment process can be divided into two groups: digestion processes, which are anaerobic and oxidation processes, which are aerobic (Voulvoulis et al., 2015, Singh et al., 2016). In the following table 2, the end products of the aerobic and anaerobic microbial degradation of the major organic substrates found in sewage are listed:
|
 There are numerous problems in trying to use nitrification to remove from wastewater, particularly in temperate or cold climates. The nitrifying bacteria are relatively slow growing and function best at temperatures of 20° – 25°. At temperatures of 5°, their metabolism may be almost dormant. Thus, although nitrification may achieve in a treatment plant in warmer weather, nitrification in cold climates may be very limited. The presence of organic matter in the wastewater is not necessary for these bacteria, but trace levels of nutrients such as phosphate, magnesium, copper, and iron are essential (Kadlec and Wallace, 2008, Kroiss et al., 1992, Ge et al., 2015). Many heavy metals and some organic will inhibit nitrification. The optimum pH for nitrification is 7.5 to 8.5, and the process is very slow if the pH drops to 6.0. The addition of small quantities of alkali may be required to maintain a suitable pH because of the acidity formed in the nitrifying process. All of the aerobic biological treatment processes can be adapted to nitrify ammonia. The main operational problems are the inability to operate the nitrifying process at low ambient temperatures and sensitivity of nitrifying bacteria to low concentrations of toxic constituents (Benbi and Richter, 2012, Li et al., 2015). DenitrificationDe-nitrification, the opposite of nitrification in water, is the removal of oxygen from nitrates in anoxic or anaerobic conditions resulting eventually in gaseous nitrogen that bubbles off and thus removed. Many heterotrophic bacteria found in an aerobic biological process can adapt to using the oxygen that is combined in nitrates if the dissolved oxygen is very low or zero (respiration under anoxic conditions). The sludge in the bottom of a secondary sedimentation tank has little oxygen and with a nitrified effluent a problem may occur if the sludge rises to the surface because of denitrification, caused by the nitrogen bubbles lifting the sludge. This is unlikely if the sludge spends less than 2h in the settling tank. The major remedy is to increase the rates of sludge collection and return from the settling tank. Sludge collection can increase by having three or four scraper arms in the tank (Zhao et al., 1999, Qian et al., 2015). In activated sludge treatment, if the sludge well nitrified, some denitrification can be achieved by not fully aerating the return sludge in the first compartment of the aeration tank. The return sludge is then efficiently mixed with the incoming from the primary sedimentation tanks. Some 50% denitrification has thus regularly achieved. Alternatively a reduced number of air diffusers can be used instead (one –third of the number ordinarily used for aeration) with equally good results. Sewage may also denitrify by locating an anoxic activated sludge plant after secondary sedimentation. Methanol or settled sewage may add to the secondary effluent. As it enters the anoxic mixing tank, to act as organic carbons source for the denitrifying bacteria. There is a further stage of sedimentation, from which the sludge returned to the anoxic mixing tank. Other schemes exist to get denitrification in activated sludge plants. For example four zones alternating anoxic, aerobic, anoxic and aerobic followed by sedimentation. Alternatively an anaerobic filter may be used to denitrify the effluent from secondary sedimentation. The same process has been observed in polluted rivers when a batch of well-nitrified effluent meets some raw sewage. The nitrogen bubbles off. Appreciable losses of nitrate by denitrification have been noticed from effluent left for a few days in a lagoon for tertiary treatment (Chai et al., 2014, Ntougias et al., 2015, Lust, 2014).
There are numerous problems in trying to use nitrification to remove from wastewater, particularly in temperate or cold climates. The nitrifying bacteria are relatively slow growing and function best at temperatures of 20° – 25°. At temperatures of 5°, their metabolism may be almost dormant. Thus, although nitrification may achieve in a treatment plant in warmer weather, nitrification in cold climates may be very limited. The presence of organic matter in the wastewater is not necessary for these bacteria, but trace levels of nutrients such as phosphate, magnesium, copper, and iron are essential (Kadlec and Wallace, 2008, Kroiss et al., 1992, Ge et al., 2015). Many heavy metals and some organic will inhibit nitrification. The optimum pH for nitrification is 7.5 to 8.5, and the process is very slow if the pH drops to 6.0. The addition of small quantities of alkali may be required to maintain a suitable pH because of the acidity formed in the nitrifying process. All of the aerobic biological treatment processes can be adapted to nitrify ammonia. The main operational problems are the inability to operate the nitrifying process at low ambient temperatures and sensitivity of nitrifying bacteria to low concentrations of toxic constituents (Benbi and Richter, 2012, Li et al., 2015). DenitrificationDe-nitrification, the opposite of nitrification in water, is the removal of oxygen from nitrates in anoxic or anaerobic conditions resulting eventually in gaseous nitrogen that bubbles off and thus removed. Many heterotrophic bacteria found in an aerobic biological process can adapt to using the oxygen that is combined in nitrates if the dissolved oxygen is very low or zero (respiration under anoxic conditions). The sludge in the bottom of a secondary sedimentation tank has little oxygen and with a nitrified effluent a problem may occur if the sludge rises to the surface because of denitrification, caused by the nitrogen bubbles lifting the sludge. This is unlikely if the sludge spends less than 2h in the settling tank. The major remedy is to increase the rates of sludge collection and return from the settling tank. Sludge collection can increase by having three or four scraper arms in the tank (Zhao et al., 1999, Qian et al., 2015). In activated sludge treatment, if the sludge well nitrified, some denitrification can be achieved by not fully aerating the return sludge in the first compartment of the aeration tank. The return sludge is then efficiently mixed with the incoming from the primary sedimentation tanks. Some 50% denitrification has thus regularly achieved. Alternatively a reduced number of air diffusers can be used instead (one –third of the number ordinarily used for aeration) with equally good results. Sewage may also denitrify by locating an anoxic activated sludge plant after secondary sedimentation. Methanol or settled sewage may add to the secondary effluent. As it enters the anoxic mixing tank, to act as organic carbons source for the denitrifying bacteria. There is a further stage of sedimentation, from which the sludge returned to the anoxic mixing tank. Other schemes exist to get denitrification in activated sludge plants. For example four zones alternating anoxic, aerobic, anoxic and aerobic followed by sedimentation. Alternatively an anaerobic filter may be used to denitrify the effluent from secondary sedimentation. The same process has been observed in polluted rivers when a batch of well-nitrified effluent meets some raw sewage. The nitrogen bubbles off. Appreciable losses of nitrate by denitrification have been noticed from effluent left for a few days in a lagoon for tertiary treatment (Chai et al., 2014, Ntougias et al., 2015, Lust, 2014). Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML