-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2016; 6(2): 33-37
doi:10.5923/j.ajee.20160602.01

Modeling Relationship between Organic Carbon Partition Coefficient and Pesticides Solubility of Pesticides used along the Shore of Lake Naivasha, Kenya
Simon Mburu Njoroge1, Thomas Mutuku Munyao2, Odipo Osano3
1Department of Environmental Health, Moi University, Eldoret, Kenya
2Department of Environmental Biology and Health, University of Eldoret, Eldoret, Kenya
3Department of Environmental Earth Science, University of Eldoret, Eldoret, Kenya
Correspondence to: Simon Mburu Njoroge, Department of Environmental Health, Moi University, Eldoret, Kenya.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Pesticides have many different properties that affect their behaviour in the environment. Pesticide’s solubility in water has a great impact on leaching potential and environmental fate. The objective of this study was to determine the relationship between organic carbon based partition coefficient (koc) and pesticides solubility (S) of pesticides used along the shore of Lake Naivasha using regression analysis. The properties (S, and soil/water equilibrium partition coefficient (kd)) of pesticides selected from an inventory of pesticides used in farms around Lake Naivasha, were determined from the manufacturers’ materials safety data sheets. The organic carbon (foc) of the soil from the study area was then determined using the loss-on-ignition (LOI) method and used to calculate koc. The results showed that the soils around Lake Naivasha had a mean organic carbon (foc) content of 1.770% and a regression equation for koc and S for the area to be log koc = -0.368logS + 3.256. It was concluded that this relationship can be used to estimate the organic carbon based partition coefficient (koc) of a pesticide where S is available, and the results compared with values determined experimentally and from other models.
Keywords: Pesticides, Groundwater contamination, Partition coefficient, Solubility, Organic carbon, Lake Naivasha
Cite this paper: Simon Mburu Njoroge, Thomas Mutuku Munyao, Odipo Osano, Modeling Relationship between Organic Carbon Partition Coefficient and Pesticides Solubility of Pesticides used along the Shore of Lake Naivasha, Kenya, American Journal of Environmental Engineering, Vol. 6 No. 2, 2016, pp. 33-37. doi: 10.5923/j.ajee.20160602.01.
Article Outline
1. Introduction
- Pesticides have many different properties that affect their behaviour in the environment. Pesticide properties include physical and chemical characteristics such as solubility, adsorption, volatility, and the potential for degradation. Some of these properties play a significant role in the contamination processes of groundwater. For a water contaminant to be available for the downward movement of the water flow, it should be water soluble, not strongly adsorbed to soil particles, resistant to biotic and abiotic degradation reactions, and not very volatile [1-3]. Other factors influencing the processes of pesticide transport are climate, soil pedology, physical and chemical conditions and soil biota and management ([4] and [5]).Pesticide’s solubility in water has a great impact on leaching potential and environmental fate. In the study of the environmental fate of organic chemicals, the octanol/ water partition coefficient (kow) has become a key parameter. It has been shown to be correlated to water solubility, soil/sediment sorption coefficient (kd), and bioconcentration [6, 7]. Of the three properties that can be estimated from kow, water solubility is the most important because it affects both the fate and transport of chemicals [8, 9]. For example, highly soluble chemicals become quickly distributed by the hydrologic cycle, have low sorption coefficients for soils and sediments, and tend to be more easily degraded by microorganisms [10, 11].Pesticide chemicals that dissolve readily in water are highly soluble and, thus, are generally carried with the water flow. Such pesticides have a tendency to leach from the soil to groundwater. As the water solubility of a pesticide increases, there is a greater likelihood it will be leached through the soil to groundwater or runoff into surface water. In general, pesticides with solubility of greater than 30 part per million have an increased tendency to leach [12]. Once a pesticide enters a soil, a portion adheres to soil particles and some remains in solution in the soil water [13]. The soil/water equilibrium partition coefficient, also known as the adsorption coefficient (kd) is defined as the ratio between the concentration in soil and the concentration in water. It can therefore be used to estimate the dissolved or the adsorbed fraction in a soil-water system for any chemical. In a study of six pesticides (bentazone, dimethoate, MCPA, pirimicarb, propiconazole and fenpropimorph), [14] it was found that the ratio of lost to applied pesticide decreased with increasing adsorption coefficient.Each pesticide behaves differently in different soil. In order to evaluate the behaviour of a pesticide at a specific site, the organic carbon normalized soil/water equilibrium partition coefficient (koc) is used. Koc provides a measure of mobility in which the effects of soil type and management history are specifically accounted for. Organic carbon content (foc) of a site (estimated to be 58 percent of the organic matter content, is used in the calculation [15, 16]. The US EPA kd/koc conversion is based on an organic matter content of 2 percent and an organic carbon content of 1.16 percent [15].
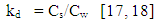 | (1) |
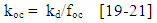 | (2) |
2. Material and Methods
2.1. Pesticide Properties
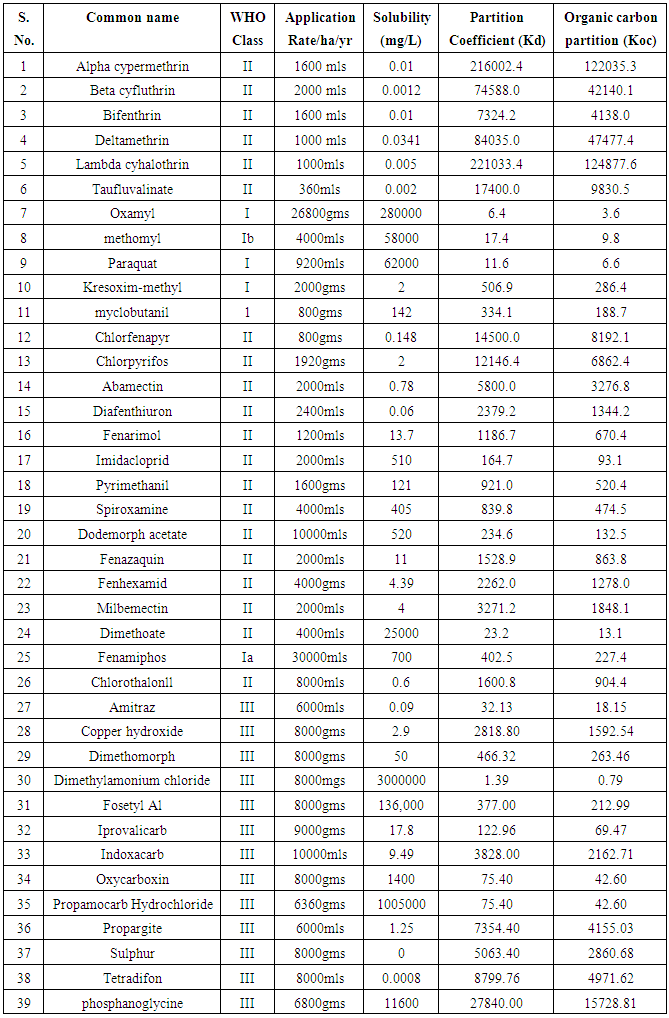
- An inventory of pesticides used in farms around Lake Naivasha was first prepared from which 39 pesticides were selected based on their WHO toxicity classification and annual rates of application (Table 1). The properties of selected pesticides (solubility (S), and soil/water equilibrium partition coefficient (kd)) were determined from the manufacturers’ materials safety data sheets and other literature. The organic carbon (foc) of the soil from the study area was then determined and used to calculate the organic carbon partition coefficient (koc).
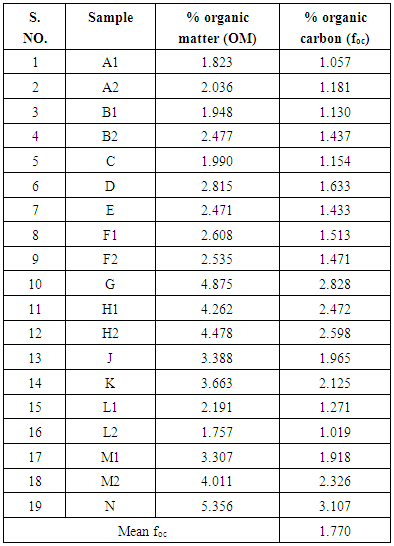
2.2. Soil Organic Carbon Determination
- Nineteen (19) soil samples for soil organic carbon analysis were conveniently sampled from farms along the shore of Lake Naivasha (Fig. 1). The soils were sampled at a depth of 15-30 cm to eliminate plant roots which could have given higher than correct values of organic matter and organic carbon. The samples were collected on different days of the week and stored at 4oC to minimize organic compounds loss. The organic matter in the samples was determined at Moi University School of Environmental Studies Analytical Laboratory using the loss-on-ignition (LOI) method [27, 28].
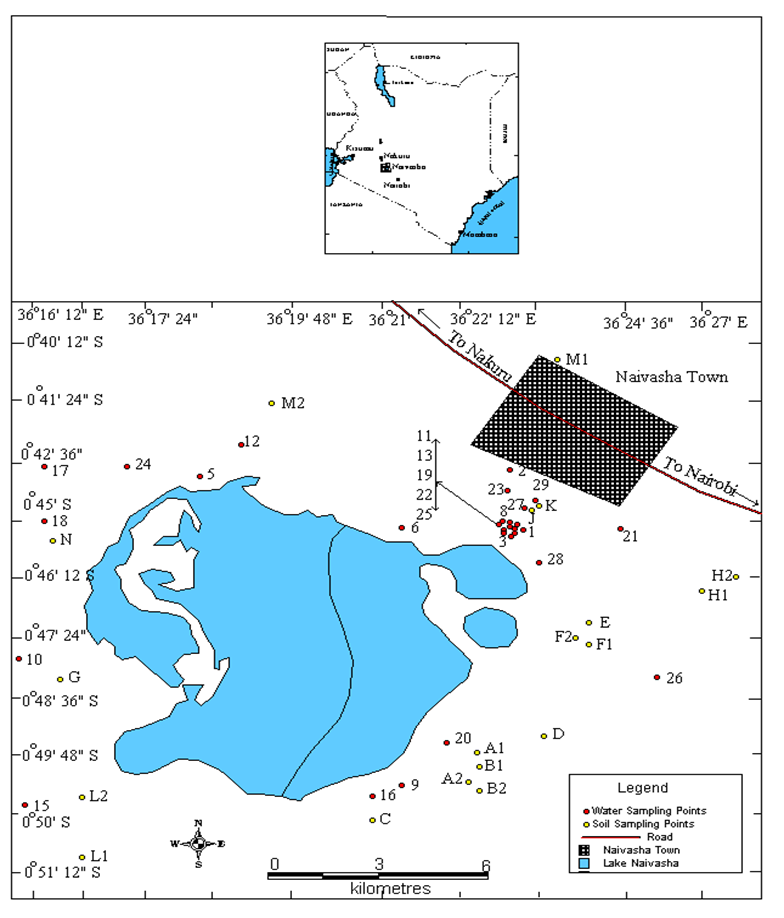 | Figure 1. Soil sampling sites |
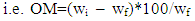 | (3) |
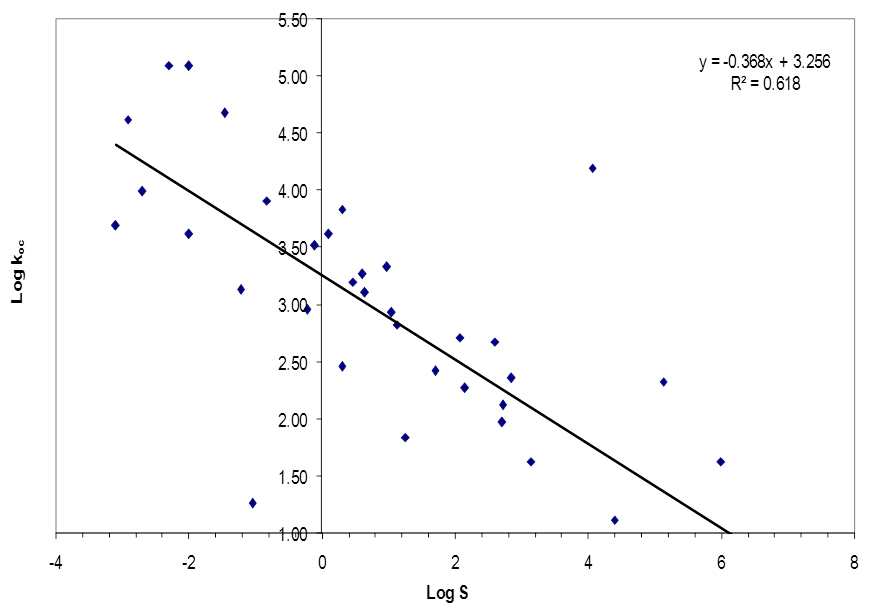 | Figure 2. Plot of Log Koc versus Log S of pesticides used on the shore of Lake Naivasha |
3. Results and Discussion
- Table 1 shows the solubility and soil/water equilibrium partition coefficient (kd) of selected pesticides. Table 2 shows the values of organic matter and organic carbon of soils around Lake Naivasha. The mean organic carbon (foc) of 1.770% (table 2 was used to calculate the organic carbon based partition coefficient (koc) of 39 pesticides shown in Table 1. Figure 2 shows a plot of the logarithm of organic carbon based partition coefficient (log koc) versus the logarithm of solubility (log S).
|
|
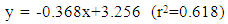 | (4) |
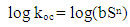 | (5) |
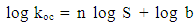 | (6) |
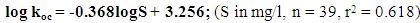 | (7) |
4. Conclusions
- The soils around Lake Naivasha had a mean organic carbon (foc) content of 1.770%. The relationship between the organic carbon based partition coefficient (koc) and solubility (S) for the area along the shores of Lake Naivasha was determined to be: log koc = -0.368logS + 3.256.This relationship can therefore be used to estimate the organic carbon based partition coefficient (koc) of a pesticide where S is available, and the results compared with values determined experimentally and from other models.
ACKNOWLEDGEMENTS
- This work is based on a PhD research by the corresponding author. The research was financially supported by the German Academic Exchange Service (DAAD).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML