Petronela Nechita
Department Department of Environmental, Applied Engineering and Agriculture, Dunarea de Jos University of Galati, Romania
Correspondence to: Petronela Nechita, Department Department of Environmental, Applied Engineering and Agriculture, Dunarea de Jos University of Galati, Romania.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The new economic and environmental requirements involve the development of low-pollution technologies and finding the new economically and environmental friendly materials which can be used in the practice of industrial wastewaters decontamination. A particular interest in this direction is assigned to the natural additives based on renewable resources, biocompatible and biodegradable that has a high ability to retain the pollutants from wastewaters. In this paper are presented the tests of some natural additives (recycled resources based on clamshells) use in the adsorption process of pollutants from textile industrial wastewaters. The results showed that the studied materials have a high capacity of dyes removal (about 80%) and retention of other pollutants such as: total suspended solids (TSS) (removal efficiency about 90%), COD (removal efficiency about 59%) and heavy metals: Lead (Pb2+) and Copper (Cu2+). This unconventional method can be a cheap and effective alternative for the treatment of industrial wastewaters, replacing in the physical-chemical treatment stage, the synthetic coagulants and activated carbon, that are very expensive and with negative impact on the environment.
Keywords:
Depollution, Industrial wastewaters, Natural additives, Adsorption, Textiles industry
Cite this paper: Petronela Nechita, Natural Additives Used in Adsorption of Pollutants from Textile Wastewaters, American Journal of Environmental Engineering, Vol. 5 No. 2, 2015, pp. 39-42. doi: 10.5923/j.ajee.20150502.01.
1. Introduction
Using the unconventional natural materials to reduce the load of pollutants from wastewaters has become an area of interest in the current research with high applicative potential. In literature are reported more and more studies and research regarding the use of natural additives in water remediation process with great efficiency of dyes, divalent metals (Fe, Ni) or heavy metals (Cu, Pb) removal, as well as of other pollutants (organic and inorganic substances) [1-5].In the last time, the using of polysaccharides (based on chitosan or cellulose derivatives) gains a high interest in removing of pollutants from wastewater (heavy metal ions, dyes, cyanides, sulphides, etc.), due to are made from renewable resources and are characterized by low carbon footprint [1, 6].Chitin (typically extracted from the shells of crabs, lobsters and shrimps) is the second biopolymer after cellulose as abundance, and its deacetylation product, chitosan is a biopolymer with unique properties known as an environmental friendly raw material, with more and more uses in water treatment processes (adsorption, coagulation-flocculation) [6, 7]. Chitosan has attracted considerable interest in wastewaters treatment, due to a unique combination of properties such as biocompatibility, biodegradability and complexing, coagulation and ions exchange capacity. The adsorption of metal ions on chitosan is based on the equilibrium reactions, so that the efficiency of adsorption will depend both on the surface properties of chitosan, as well as the experimental conditions of work. In the adsorption of metal ions on chitosan are involved the functional groups on the sorbent surface. The interactions between these functional groups and metal ions are most likely electrostatic or complexation type. The metal ions near functional groups tend to interact with active centres that have the most availability (geometric), and once these active centres are busy, there are steric hindrance which greatly limits the penetration of other ions from solution to free functional groups [6]. Clam shells (mussels, scallops) are able to remove the metal ions from wastewater as result of aragonite presence, a biogenic form of calcium carbonate which has the ability to change the calcium ions with heavy metal ions. Furthermore, by calcination at 600°C - 800°C temperature, the clam shells have a higher adsorption efficiency for the other pollutants, also, since the calcium carbonate is converted into calcium oxide [8]. The reactivity of various types of natural calcium carbonate decreases as follow: Dolomite < Chalk < Marble < Clam shells.
2. Experimental
2.1. Materials
Industrial wastewater used in experimental program was collected according to ISO 5667/1992 method, from manufacturing and finishing of textile fibers. Wastewater sample was stored in a refrigerator at about 4°C.Clam shells – waste from the Black Sea, used as natural additives, were grinded for 5 hours in ceramic balls mill (diameter of balls = 1 cm) and then calcined in a furnace for 1h at 800°C (Figure 1). | Figure 1. Clamshells: a) initial; b) after grinding; c) after grinding and calcination |
Size distribution of clamshells particles was determined in the laboratory using dry classifying on wires with mesh size of 500μ, 200μ, 100μ, 50μ and 20μ. The medium size of clamshells particles was between 100μ and 500μ (Figure 2).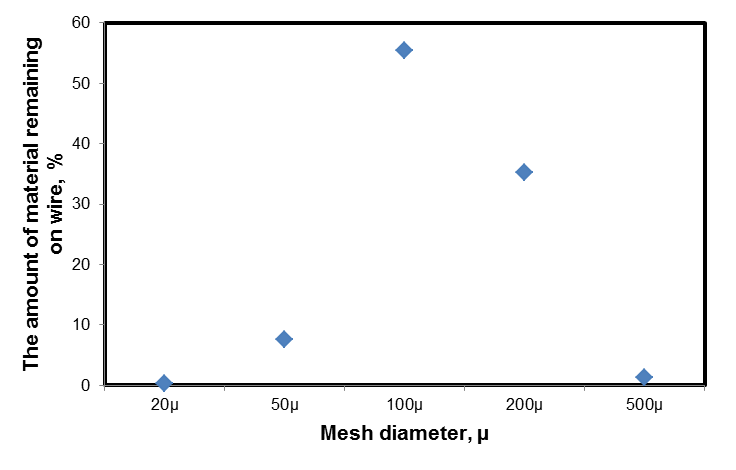 | Figure 2. Size distribution of clamshells particles |
Synthetic additives - resin type Purolite C100MB, with the following characteristics: fine particulate material (0,42 – 1,2 mm), pH range : 0 – 14, functional group: R – SO3. (figure 3) | Figure 3. Synthetic resin - Purolite C100MB |
2.2. Methods
The textile industrial wastewater was treated with clamshells and synthetic additives using the static adsorption method, based on the following procedure: in an Erlenmeyer flask were placed 100 ml wastewater to which was added 1g of additive. The mixture was stirred for 60 minutes and then filtered through a filter paper.The wastewater quality was controlled before and after additives treatment by measuring of the following pollution parameters: total suspended solids (TSS), chemical oxygen demand COD, heavy metal ions (Cu (II), Pb (II)), sulphides, chloride, greases content, dye content, pH, using analytical methods (gravimetric, colorimetric and spectrophometric).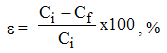 | (1) |
Where: Ci – pollutant content before treatment, [mg/l] Cf – pollutant content after treatment, [mg/l]
3. Results and Discussions
The textile industry uses large amounts of water for dying and washing. Textile wastewater is generally alkaline and high in pigments, BOD and suspended solids [7]. Thus, the colour of wastewater samples is over 50° Pt/Co. Figure 3 presents comparative results on the efficiency of removal from wastewater pollutants using as adsorption material, calcinated and uncalcinated clamshells.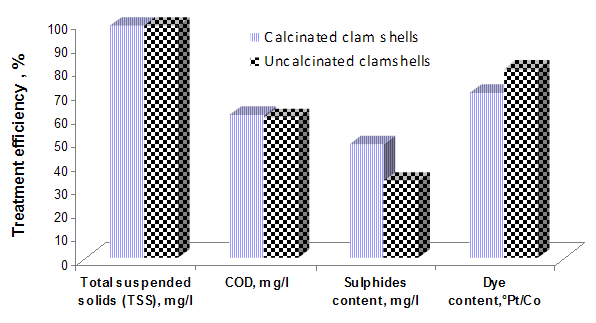 | Figure 3. The adsorbtion efficiency versus additive grade |
Analysing the above presented results can be seen the high efficiency of pollutants removal from wastewater, especially for the total suspended solids and colour. In both cases, it is observed that the adsorption process is an effective method of dyes removing from wastewater, registering a higher efficiency when the calcined clamshells were used. Regarding the adsorption of heavy metal ions from wastewater, there is a better removal of Pb (II) in the case of uncalcined clamshells (figure 4).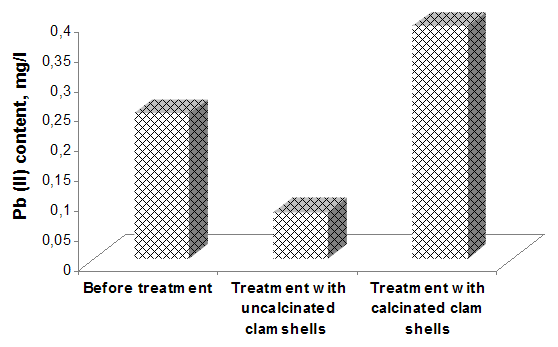 | Figure 4. Removal efficiency of Pb(II) ions versus additive grade |
Clamshells are able to effectively remove the heavy metal ions, due to the presence of aragonite, a biogenic form of calcium carbonate which has the ability to change the calcium ions with heavy metal ions. The difference between the calcinated and uncalcinated clamshells regarding the removal efficiency of Pb (II) ions is due to their composition; by calcination at 600°C - 800°C, calcium carbonate of clamshells is converted into calcium oxide with a lower complexation capacity of the metal ions.Regarding the pH influence was observed that the wastewater treated with clamshells has changed slightly pH from 7.65 to 8.0 in the case of uncalcined clamshells, and from 7.65 to 8.5 when calcined shells were used. Aiming to assess the effect of pH on removal efficiency of pollutants from the wastewater, tests were carried out at three pH levels: 2, 4 and 8. (Figures 5, 6 and 7)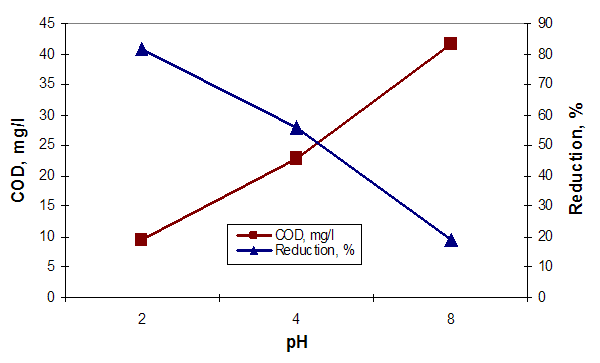 | Figure 5. The influence of pH on the COD and removing efficiency |
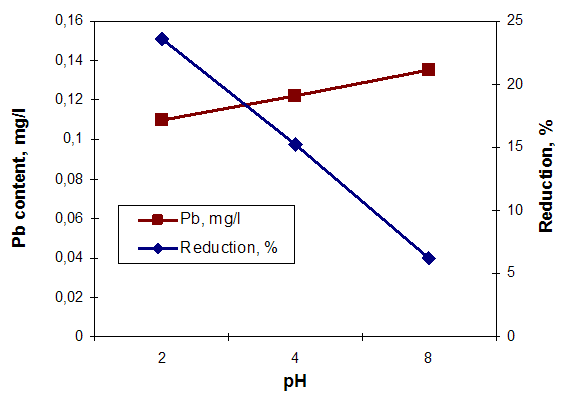 | Figure 6. The influence of pH on the Pb (II) content and removing efficiency |
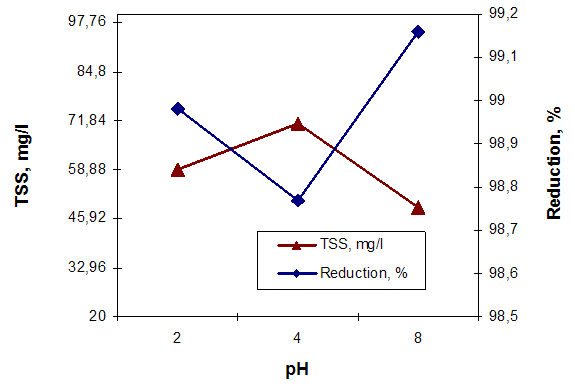 | Figure 7. The influence of pH on the TSS content and removing efficiency |
Analyzing the graphs of Figures 5, 6 and 7 it can make the following observations: the highest removal efficiency of oxidable pollutants and Pb content (II) is obtained at pH = 2; the efficiency of total suspended solids removal presents higher values at pH = 8. These changes are due on the one hand, of the high reactivity of calcium carbonate from clamshells and on the other hand, the fact that at acid pH conditions, the dissolution of calcium carbonate occurs. These leads to release of large amounts of ions Ca2+ that are able to replace the heavy metal ions. The low retention of TSS content, can be explained by formation of large amounts of CO2 at acid pH conditions, that cover the calcium carbonate particles, decreasing their filtering capacity [6, 9]. Comparing the results obtained by using the clamshells for removing of wastewater pollutants with those obtained in treatments with synthetic resin, can be observed that the Purolite C100 resin has no effect on the wastewater dye removing. (figure 8, b) and figure 9). Regarding the efficiency removing of TSS, COD, grease content and Cu (II) can be observed that the Purolite C 100 has the similar results with natural additives based on clamshells. (figure 8, a), b)).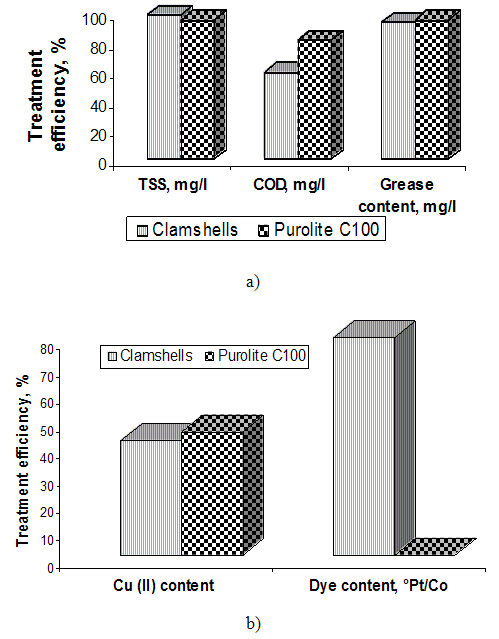 | Figure 8. The influence of additive grade on the treatment efficiency of wastewater |
 | Figure 9. The evolution of wastewater colour: a) without treatment; b) treatment with clamshells; c) treatment with Purolite C100 resin |
4. Conclusions
Based on the obtained results regarding the adsorption of pollutants from industrial wastewaters using the natural additives (such as waste based on clamshells) the following conclusions can be formulated:● use the natural additives based on clamshells in the static adsorption process of wastewater pollutants has a result a high efficiency of the dyes, organic impurities, TSS, and heavy metals (Pb(II)) removal;● the clamshells are effectively on the metal ions removing, due to the presence of aragonite, a biogenic form of calcium carbonate, which has the ability to exchange the calcium ions with heavy metal ions;● the difference between calcinated and uncalcinated clamshells regarding the removal capacity of Pb (II) and other pollutants, is due to their composition; thus, by calcination at 600°C - 800°C, calcium carbonate from clamshells turns into CaO with a lower capacity of heavy metal ions complexing;● the adsorption process is influenced by the pH, a higher efficiency of organic and heavy metal pollutants removing occurring at pH 2; for the TSS the optimum efficiency was registered at pH 8; ● based on obtained results, the clam shells can be an alternative to synthetic resin (Purolite C100) in wastewater treatments.
References
| [1] | Farkas, A.E. et.al, (2013), “Polysaccharides utilization for water decontamination”, Proceedings of The 7th International Symposium on Advanced Technologies for the Pulp, Paper and Corrugated Board Industry, Brăila, Romania. |
| [2] | Şuteu, D. et al (2013), “Industrial cellolignine wastes as adsorbent for removal of Methylen blue dye from aqueous solutions”, BioResources, 8(1), 427-446. |
| [3] | Lupea, V.M., (2012) “Studii privind utilizarea algelor marine ca sorbent low-cost pentru îndepărtarea ionilor metlici din apele uzate”, Doctoral Thesis, Iaşi, Romania (in Romanian). |
| [4] | Gupta, V.K., et al., (2009), “Application of low-cost adsorbents for dye removal – A review”, Journal of Environmental Management 90, 2313–2342. |
| [5] | Tudor, H.E.A., Gryte, C.C., Harris, C.C., (2006), “Sea shells: Detoxifying agents for metal-contaminated waters”, Water, Air, and Soil Pollution, 173: 209–242. |
| [6] | Asandei, D., Bulgariu L., Bobu E. (2010) “Removal of Cu(II) ions from aqueous solutions by adsorption on chitosan” – Proceedings of 14th International Symposium on Cellulose Chemistry and Technology, Iaşi, Romania. |
| [7] | Mohd Arrifin, A.H., Tan Pei, Li., Zainura, Z.N., (2009), “Coagulation and flocculation treatment of wastewater in textile industry using chitosan”, Journal of Chemical and Natural Resources Engineering, Vol.4(1):43-53. |
| [8] | Farhah, A. I., Ahmad Z. A., (2013), “Experimental determination of Cd2+ adsorption mechanism on low-cost biological waste”, Front. Environ. Sci. Eng. 7(3): 356–364. |
| [9] | Sari, A., Tuzen, M., (2009), “Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass”, J Hazard Mater. May 30; 164(2-3):1004-11. |



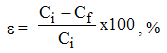







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML