| [1] | W. De los Santos Ramosa, T. Poznyaka, I. Chairezb, I. Córdova R., 2009, Remediation of lignin and its derivatives from pulp and paper industry wastewater by the combination of chemical precipitation and ozonation., Journal of Hazardous Materials, pp. 428–434. |
| [2] | Hocking, M. B. Production of and paper. 453–504, s.l. : Handbook of Chemical Technology and Pollution Control (Third Edition), 2005. |
| [3] | D. Pokhrel, T. Viraraghavan, 2004, Treatment of pulp and paper mill wastewater—a review, Science of the Total Environment, Vol. 333, 37– 58. |
| [4] | M.C. Area, J.L. Valade, 1998 Revisión de los procesos de pulpado con acción química, El papel. La revista papelera para Espeña y América Latina, Vol. 69, 47-51. |
| [5] | Muna Ali, T.R. Sreekrishnan, 2011, Aquatic toxicity from pulp and paper mill effluents: a review, Advances in Environmental Research, Vol. 5, 175-196. |
| [6] | B. Karrasch, O. Parra, H. Cid, M. Mehrens, P. Pacheco, R. Urrutia, C. Valdovinos, C. Zaro., 2006, Effects of pulp and paper mill effluents on the microplankton and microbial self-purification capabilities of the Biobío River, Chile. Science of The Total Environment, Volume 359, Issues 1–3, Vol. 359, 194–208 (1-3). |
| [7] | T.G Kovacs, P.H Martel, R.H Voss, 2002, Assessing the biological status of fish in a river receiving pulp and paper mill effluents, Environmental Pollution, Vol. 118 (1), 123–140. |
| [8] | Integrated Pollution Prevention and Control (IPPC) Reference Document on Best Available Techniques in the Pulp and Paper Industry. EUROPEAN COMMISSION. 2001. |
| [9] | A. Ouchi, A. Saruwatari, T. Suzuki, 2008, An efficient photochemical bleaching of kraft pulps using total halogen-free reducing reagents, Journal of Photochemistry and Photobiology A: Chemistry, Vols. 193 (2-3), 122–128. |
| [10] | M. Mohamed, M. Matayun, T. S. Lim, 1989, Chlorinated organics in tropical hardwood kraft pulp and paper mill effluents and their elimination in an activated sludge treatment system, Pertanika, Vol. 2(3), 387- 394. |
| [11] | M.C. Area, S. A. Ojeda, O. M. Barboza, D. I. Bengoechea, F. F. Felissia, 2010, Tratamientos aplicables para la reducción de la DQO recalcitrante de efluentes de pulpado quimimecánicos y semiquímicos. (Revisión)., Rev. Cien. Tecnol., Vol. 13, 4-10. |
| [12] | E. Magnus, G.E. Carlberg, H.H. Norske, 2000, TMP wastewater treatment including a biological high-efficiency compact reactor., Nord. Pulp Pap. Res. J, Vol. 15 (1), 29–36. |
| [13] | C. S. Tripathi, D. G. Allen, 1999,Comparison of mesophilic and thermophilic aerobic biological treatment in sequencing batch reactors treating bleached kraft pulp mill effluent, Water Research, Vol. 33 (3), 836–846. |
| [14] | Bajpai, P. Treatment of pulp and paper mill effluents with anaerobic technology, Randalls Road, Leatherhead, Pira International, 2000. |
| [15] | A Kostamo, B Holmbom, J.V.K Kukkonen, 2004, Fate of wood extractives in wastewater treatment plants at kraft pulp mills and mechanical pulp mills, Water Research, Vol. 38, 972–982. |
| [16] | M.C Diez, G Castillo, L Aguilar, G Vidal, M.L Mora, 2002, Operational factors and nutrient effects on activated sludge treatment of Pinus radiata kraft mill wastewater, Bioresource Technology, Vol. 83 (2), 131–138. |
| [17] | T. Liu, H. Hu, Z. He, Y. Ni, 2011, Treatment of poplar alkaline peroxide mechanical pulping (APMP) effluent with Aspergillus niger, Bioresource Technology, Vol. 102 (15), 7361–7365. |
| [18] | A.P. Buzzini, E.P. Gianotti, E.C. Pire, 2005, UASB performance for bleached and unbleached kraft pulp synthetic wastewater treatment., Chemosphere, Vol. 59 (1), 55–61. |
| [19] | I. Akmehmet Balcıoğlu, E. Tarlan, C. Kıvılcımdan, M. Türker Saçan, 2007, Merits of ozonation and catalytic ozonation pre-treatment in the algal treatment of pulp and paper mill effluents, Journal of Environmental Management, Vol. 85 (4), 918–926. |
| [20] | Kyoung-Hun Kim, Son-Ki Ihm, 2011, Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review, Journal of Hazardous Materials., Vol 186 (1), 16–34. |
| [21] | A. Marco, S. Esplugas, G. Saum, 1997, How and why combine chemical and biological processes for wastewater treatment, Water Sci. Technol, Vol. 35 (4), 321–327. |
| [22] | J.P. Scott, D.F. Ollis, 1995, Integration of chemical and biological oxidation processes for water treatment: review and recommendations, Environ. Prog., Vol. 14, 88–103. |
| [23] | S.h Karimi, A. Abdulkhani, A. H. B. Ghazali, F. Ahmadun, A. Karimi, 2009, Color remediation of chemimechanical pulping effluent using combination of enzymatic treatment and Fenton reaction, Desalination, Vol. 249, 870–877. |
| [24] | A.C. Rodrigues, M. Boroski, N.S. Shimada, J.C. Garcia, J. Nozakim, N. Hioka, 2008, Treatment of paper pulp and paper mill wastewater by coagulation-flocculation followed by heterogeneous photocatalysis, J. Photochem. Photobiol. A: Chem., Vol. 194, 1-10. |
| [25] | P. R. Meza, F. E. Felissia, M. C. Area, 2010, Reduction of the recalcitrant cod of high yield pulp mills effluents by aop. part 1. combination of ozone and activated sludge, BioResources, Vol. 6 (2), 1053-1068. |
| [26] | L Larrea, C.F Forster, D Melé, 1989, Changes in lignin during diffused air activated sludge treatment of kraft effluents, Water Research, Vol. 3 (9), 1073–1080. |
| [27] | A.P Buzzini, I.K. Sakamoto, M.B. Varesche, E.C. Pires. Evaluation of the microbial diversity in an UASB reactor treating wastewater from an unbleached pulp plant, Process Biochemistry, 2006, Vol. 41 (1), 168–176. |
| [28] | G. Thompson, J. Swain, M. Kay, C.F. Forster, 2001, The treatment of pulp and paper mill effluent: a review, Bioresource Technology, Vol. 77, 275-286. |
| [29] | L. M. da Silva, W. F. Jardim, 2006, Trends and strategies of ozone application in environmental problems, Quim. Nova, Vol. 29 (2), 310-317. |
| [30] | Basheer Hasan Diya’uddeen, Wan Mohd Ashri Wan Daud, A.R. Abdul Aziz, 2011, Treatment technologies for petroleum refinery effluents: A review, Process Safety and Environmental Protection, Vol. 89 (2), 95–105. |
| [31] | C. A. Martínez-Huitle, E. Brillas, 2009, Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review, Applied Catalysis B: Environmental, Vols. 87 (3-4), 105–145. |
| [32] | R. Alnaizy, A. AkgermanU, 2000, Advanced oxidation of phenolic compounds, Advances in Environmental Research, Vol. 4, 233 -244. |
| [33] | J. Beltran De Heredia, J. Torregrosa, J. R. Dominguez, J. A. Pere, 2001, Kinetic model for phenolic compound oxidation by Fenton's reagent, Chemosphere, Vol. 45 (1), 85–90. |
| [34] | R. Andreozzi, V. Caprio, A. Insola, R. Marotta, 1999, Advanced oxidation processes (AOP) for water purification and recovery., Catalysis Today, Vol. 53, –59. |
| [35] | I. Munoz, J. Rieradevall, F. Torrades, J.Peral, X. Domenech, 2006, Environmental assessment of different advanced oxidation processes applied to a bleaching Kraft mill effluent, Chemosphere, Vol. 62, 9–16. |
| [36] | Kati Eskelinen, Heikki Särkkä, Tonni Agustiono Kurniawan, Mika E.T. Sillanpää, 2010, Removal of recalcitrant contaminants from bleaching effluents in pulp and paper mills using ultrasonic irradiation and Fenton-like oxidation, electrochemical treatment, and/or chemical precipitation: A comparative study, Desalination, Vols. 255 (1-3), 179–187. |
| [37] | T. A. Kurniawan, WH. Lo, 2006, Degradation of recalcitrant compounds from stabilized landfill leachate using a combination of ozone-GAC adsorption treatment, Journal of Hazardous Materials, Vol. B137, 443–455. |
| [38] | R. Matta, K. Hanna, S. Chiron, 2007, Fenton-like oxidation of 2,4,6-trinitrotoluene using different iron minerals, Science of the Total Environment, Vol. 385, 242-251. |
| [39] | Munter, R., 2001, Advanced oxidation processes-current status and prospects, Proc. Est.Acad. Sci. Chem, Vol. 50, 59–80. |
| [40] | W. H. Glaze, Joon-Wun Kang, 1989, Advanced Oxidation Processes. Description of a Kinetic Model for theOxidation of Hazardous Materials in Aqueous Media with Ozone and Hydrogen Peroxide in a Semibatch Reactor, Ind. Eng. Chem. Res., Vol. 28, 1573-1580. |
| [41] | C.P. Huang, C. Dong, Z. Tang, 1993, Advanced chemical oxidation: Its present role and potential future in hazardous waste treatmen, Waste Management, Vols. 13 (5-7), 361–377. |
| [42] | Kyoung-Hun Kim, Son-Ki Ihm, 2011, Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review, Journal of Hazardous Materials, Vol. 186 (1), 16–34. |
| [43] | M. E.T. Sillanpää, T. A. Kurniawan, W. Lo, 2011, Degradation of chelating agents in aqueous solution using advanced oxidation process (AOP), Chemosphere, Vol. 83 (11), 1443–1460. |
| [44] | G. Del Moro, A. Mancini, G. Mascolo, C. Di Iaconi, , 2013, Comparison of UV/H2O2 based AOP as an end treatment or integrated with biological degradation for treating landfill leachates, Chemical Engineering Journal, Vol. 218, 133–137. |
| [45] | C.B. Chidambara Raj, Han Li Quen, 2005, Advanced oxidation processes for wastewater treatment: Optimization of UV/H2O2 process through a statistical technique, Chemical Engineering Science, Vol. 60 (19), 5305–5311. |
| [46] | M. C. Yeber, J. Rodríguez, J. Baeza, J. Freer, C. Zaror, N. Durán, H. D. Mansilla, 1999, Toxicity abatement and biodegradability enhancement of pulp mill bleaching effluent by advanced chemical oxidation, Water Science and Technology, Vols. 40 (11-12), 337–342. |
| [47] | S. Kommineni, J. Zoeckler, A. Stocking, S. Liang, A. Flores, M. Kavanaugh. Advanced oxidation processes. In: national water research institute. 111-208, 2008. |
| [48] | Sandip Sharma, J.P.Ruparelia, Manish L.Patel. A general review on Advanced Oxidation Processes for waste water treatment. 382 481, Institute of Technology, Nirma University, 2011. |
| [49] | Tratamientos avanzados de aguas residuales. Informe de vigilancia tecnológica Madrid. Universidad de Alcala, Círculo de Innovación en Tecnologías Mediomabientales y Energía (CITME). Villar, F. s.l. : Colección vt2, 2006, Vol. Cap 3. |
| [50] | Ai Ni Soon, B.H. Hameed, 2011, Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process, Desalination, Vol. 269, 1-16. |
| [51] | E. Cokay Catalkaya, F. Kargi, 2007, Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: A comparative study, Journal of Hazardous Materials, Vol. B 139, 244–253. |
| [52] | I. Muñoz, J. Rieradevall, F. Torrades, J. Peral, X. Domènech, 2006, Environmental assessment of different advanced oxidation processes applied to a bleaching Kraft mill effluen, Chemosphere, Vol. 62, 9-16. |
| [53] | M. Xu, Q. Wang, Y.i Hao, 2007, Removal of organic carbon from wastepaper pulp effluent by lab-scale solar photo-Fenton process, Journal of Hazardous Materials, Volume 148, Issues 1–2, Vols. 148 (1-2), 103–109. |
| [54] | A.M. Amat, A. Arques, M.A. Miranda, F. López, 2005, Use of ozone and/or UV in the treatment of effluents from board paper industry, Chemosphere, Vol. 60 (8), 1111–1117. |
| [55] | H.-J. Oeller, I. Demel, G. Weinberge, , 1997, Reduction in residual COD in biologically treated paper mill effluents by means of combined ozone and ozone/UV reactor stages, Water Science and Technology, Volume 35, Vols. 32 (2-3), 269–276. |
| [56] | M. F. Sevimli, E. Deliktas, S. Sahinkaya, D. Guclu, 2013, A comparative study for treatment of white liquor by different applications of Fenton process. In press: Arabian Journal of Chemistry. |
| [57] | N. Merayo, D. Hermosilla, L. Blanco, L. Cortijo, A. Blanco, 2013, Assessing the application of advanced oxidation processes, and their combination with biological treatment, to effluents from pulp and paper industry, Journal of Hazardous Materials, Vol. 263, 420– 427. |
| [58] | S. Ledakowicz, M. Michniewicz, A. Jagiella, J. Stufka-Olczyk, M. Martynelis, 2006, Elimination of resin acids by advanced oxidation processes and their impact on subsequent biodegradation, Water Research, Vol. 40, 3439-3446. |
| [59] | F. Torrades, S. Saiz, J. García-Hortal, 2011, Using central composite experimental design to optimize the degradation of black liquor by Fenton reagent, Desalination, Vol. 268, 97–102. |
| [60] | F. Torrades, M. Perez, H. D. Mansilla, J. Peral, , 2003, Experimental design of Fenton and photo-Fenton reactions for the treatment of cellulose bleaching effluents, Chemosphere, Vol. 53, 1211–1220. |
| [61] | M. Pérez, F. Torrades, J. A. Garcı́a-Hortal, X. Domènech, J. Peral, 2002, Removal of organic contaminants in paper pulp treatment effluents under Fenton and photo-Fenton conditions, Applied Catalysis B: Environmental, Vol. 36 (1), 63–74. |
| [62] | I. Muñoz, J. Rieradevall, F. Torrades, J. Peral, X. Domenech, 2006, Environmental assessment of different advanced oxidation processes applied to a bleaching Kraft mill effluent, Chemosphere, Vol. 62, 9-16. |
| [63] | M. El-Din, D. W. Smith, F. Al Momani, W. Wang, 2006, Oxidation of resin and fatty acids by ozone: Kinetics and toxicity study., sWater Research, Vol. 40 (2), 392–400. |
| [64] | N. Azbar, T. Yonar, K. Kestioglu, 2004, Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. 35-43, s.l. : Chemosphere, Vol. 55. |
| [65] | Rip G. Rice, A. Netzer. Handbook of Ozone Technology and Applications. s.l. : Ann Arbor Science, 1982. |
| [66] | A. Alvares, C. Diaper, S. Parsons, 2001, Partial oxidation by ozone to remove recalcitrance from wastewaters—a review., Environ. Technol, Vol. 22, 409–427. |
| [67] | F. Gokcen, T.A. Ozbelge. Pre-ozonation of aqueous azo dye (Acid Red-151) followed by activated sludge process, Chem. Eng. J, 2006, Vol. 123, 109–115. |
| [68] | A. Chin, P.R. Berube. Removal of disinfection by-product precursors with ozone-UV advanced oxidation process, Water Research, 2005, Vol. 39, 2136–2144. |
| [69] | V.O. Abramov, O.V. Abramov, A.E. Gekhman, V.M. Kuznetsov, G.J. Price, 2006, Ultrasonic intensification of ozone and electrochemical destruction of 1,3-dinitrobenzene and 2,4-dinitrotoluene, Ultrason. Sonochem, Vol. 13, 303–307. |
| [70] | B. Cuiping, X. Xianfeng, G Wenqi, F. Dexin, X. Mo, G. Zhongxue, X. Nian., 2011, Removal of rhodamine B by ozone-based advanced oxidation process, Desalination, , 84-90. |
| [71] | S. S. Abu Amr, H. A. Aziz, M. N. Adlan, M. J. Bashir, 2013, Pretreatment of stabilized leachate using ozone/persulfate oxidation process, Chemical Engineering Journal, Vol. 221, 492–499. |
| [72] | M.R. Assalin, M.A. Rosa, N. Durán, 2004, Remediation of Kraft effluent by ozonation: effect of applied ozone concentration and initial pH, Ozone: Science and Engineering, Vol. 26, 317–322. |
| [73] | M.-O. Buffle, U. von Gunten, 2006, Phenols and amine induced HO generation during the initial phase of natural water ozonation., Environmental Science Technology, Vol. 40 (9), 3057–3063. |
| [74] | A.M. Amat, A. Arques, M.A. Miranda, F. López, 2005, Use of ozone and/or UV in the treatment of effluents, Chemosphere, Vol. 60, 1111–1117. |
| [75] | T. Kreetachat, M. Damrongsri, V. Punsuwon, P. Vaithanomsat, 2007, Effects of ozonation process on lignin-derived compounds, Journal of Hazardous Materials, Vol. 142, 250–257. |
| [76] | Fahmi, W. Nishijima, M. Okada, 2003, Improvement of DOC removal by multi-stage AOP-biological treatment, Chemosphere, Vol. 50, 1043–1048. |
| [77] | V. Fontanier, V. Farines, J. Albet, S. Baig, J. Molinie, 2006, Study of catalyzed ozonation for advanced treatment of pulp and paper mill effluents, Water Research, Vol. 40 (2), 303–310. |
| [78] | Chun-Han Ko, Po-Hung Hsieh, Meng-Wen Chang, Jia-Ming Chern, Shih-Min Chiang, Chewn-Jeng Tzeng, 2009, Kinetics of pulp mill effluent treatment by ozone-based processes., Vols. 168 (2–3), 875–881. |
| [79] | M. Mänttäri, M. Kuosa, J. Kallas, M. Nyström, 2008, Membrane filtration and ozone treatment of biologically treated effluents from the pulp and paper industry, Journal of Membrane Science, Vols. 309 (1-2), 112–119. |
| [80] | A. Laari, S. Korhonen, T. Tuhkanen, S. Verenich, J. Kalla, 1999, Ozonation and wet oxidation in the treatment of thermomechanical pulp (TMP) circulation waters, Water Sci. Technol, Vols. 40 (11–12), 51–58. |
| [81] | R. S. Freire, A. Kunz, N.Duran, 2000, Some chemical and toxicological aspects about paper mill effluent treatment with ozone, Environ. Technol, Vol. 21, 717–721. |
| [82] | L. Bijan, M. Mohsen, 2005, Integrated ozone and biotreatment of pulp mill effluent and changes in biodegradability and molecular weight distribution of organic compounds, Water Research, Vol. 39 (16), 3763–3772. |
| [83] | A. Mounteer, J. Mokfienski, F. Amorim. Remoção de Matéria Orgânica Recalcitrante de Efluentes de Celulose Kraft de Branqueamento por Ozonólise, O Papel, Vol. 66, 64-70. |
| [84] | P. Massa, A. Dafinov, F. Medina Cabello, R. Fenoglio, 2009, Catalytic wet peroxide oxidation of phenolic solutions over Fe2O3/CeO2 and WO3/CeO2 catalyst systems, Catalysis Communications, Vol. 9, 1533–1538. |
| [85] | N. Inchaurrondo, J. Cechini, J. Font, P. Haure, 2012, Strategies for enhanced CWPO of phenol solutions., Applied Catalysis B: Environmental, Vols. 111– 112, 641– 648. |
| [86] | Ince, N. H, 1999, "Critical" effect of hydrogen peroxide in photochemical dye degradation, Water Research, Vol. 33 (4), 1080–1084. |
| [87] | S.J. Masten, S.H.R. Davies, 1994, The use of ozonation to degrade organic contaminants in wastewaters, Environ. Sci. Technol., Vol. 28, 180–185. |
| [88] | F. J. Beltran, J. M. Encinar, J. F. González, 1997, Industrial wastewater advanced oxidation. Part 2. Ozone combined with hydrogen Peroxide or UV radiation, Wat. Res., Vol. 31 (10), 2415-2428. |
| [89] | C. Zwiener, F.H. Frimme, 2000, Oxidative treatment of pharmaceuticals in water, sWater Research, Vol. 34 (6), 1881–1885. |
| [90] | P.Massa, A.Dafinov , R. Fenoglio , F. Medina Cabello, 2009, Degradación catalítica de fenol con peróxido de Hidrógeno: sistemas “Fenton heterogéneo”, Rev Soc Quím Perú. 75 (2), Vol. 75 (2), 194-200. |
| [91] | S. Dogruel, T. Olmez-Hanci, Z. Kartal, I. Arslan-Alatona, D. Orhon, 2009, Effect of Fenton’s oxidation on the particle size distribution of organic carbon in olive mill wastewater, Water Research, Vol. 43, 3974-3983. |
| [92] | L. Passauer, K. Fischer, F. Liebner, , 2011, Activation of pine kraft lignin by Fenton-type oxidation forcross-linking with oligo(oxyethylene) diglycidyl ether., Holzforschung, Vol. 65, 319–326. |
| [93] | L.F. Liotta, M. Gruttadauria, G. Di Carloc, G. Perrini, V. Librando, 2009, Heterogeneous catalytic degradation of phenolic substrates: Catalysts activity, Journal of Hazardous Materials, Vol. 162, 588–606. |
| [94] | M. S. Lucas, J. A. Peres, 2006, Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation, Dyes and Pigments, Vol. 71 (3), 236–244. |
| [95] | H. Kusic, A. Loncaric Bozic, N. Koprivanac, , 2007, Fenton type processes for minimization of organic content in coloured wastewaters: Part I: process optimization, Dyes Pigments, Vol. 74, 380–387. |
| [96] | J.A. Zazo, J.A. Casas, A.F. Mohedano, J.J. Rodríguez, 2009, Semicontinuous Fenton oxidation of phenol in aqueous solution A kinetic study, Water Research, Vol. 43, 4063–4069. |
| [97] | N.S. Inchaurrondo, P. Massa, R. Fenoglio, J. Font, P. Haure, 2012, Efficient catalytic wet peroxide oxidation of phenol at moderate temperature using a high-load supported copper catalyst, Chemical Engineering Journal, Vols. 198–199, 426–434. |
| [98] | S.G. Schrank, H.J. Jose, R.F.P.M. Moreira, H.Fr. Schroder, 2005, Applicability of Fenton and H2O2/UV reactions in the treatment of tannery wastewaters, Chemosphere, Vol. 60, 644–655. |
| [99] | Y. Xie, Z. Xiao, B. Goodell, J. Jellison, H. Militz, C. Mai, 2010, Degradation of wood veneers by Fenton’s reagents: Effects of wood constituents and low molecular weight phenolic compounds on hydrogen peroxide decomposition and wood tensile strength loss, Holzforschung, Vol. 64, 375–383. |
| [100] | V. Kavitha, K. Palanivelu, 2004, The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol, Chemosphere, Vol. 55, 1235–1243. |
| [101] | B. Bianco, I. De Michelis, F. Vegliò, 2011, Fenton treatment of complex industrial wastewater: Optimization of process conditions by surface response method, Journal of Hazardous Materials, Vol. 186, 1733–1738. |
| [102] | P. Verma, C. Mai, 2010, Hydrolysis of cellulose and wood powder treated with DMDHEU by a hydrolase enzyme complex, Fenton’s reagent, and in a liquid culture of Trametes versicolor, Holzforschung, Vol. 6, 69–75. |
| [103] | P. R Gogate, A. B Pandi, 2004, A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions.., Advances in Environmental Research, Vols. 8 (3-4), 501–551. |
| [104] | Rodríguez, D. Contreras, C. Parra, J. Freer, J. Baeza, N. Durán, 1999, Pulp mill effluent treatment by Fenton-type reactions catalyzed by iron complexes. J.,: Water Science and Technology, Vols. 40 (11-12), 351–355. |
| [105] | E.Ç. Çatalkaya, F. Karg, 2008, Advanced oxidation treatment of pulp mill effluent for TOC and toxicity removals, J. Environ. Manage., Vol. 87 (3), 396–404. |
| [106] | E. G. Solozhenko, N. M. Soboleva, V. V. Goncharuk, 1995, Decolourization of azodye solutions by fenton's oxidation., Wat. Res., Vol. 29 (9), 2206-2210. |
| [107] | Gema Pliego, Juan A. Zazo, Jose A. Casas, Juan J. Rodriguez, 2013, Case study of the application of Fenton process to highly polluted wastewater from power plant, Journal of Hazardous Materials, Vols. 252-253, 180–185. |
| [108] | M. Vilve, A. Hirvonen, M. Sillanpää, 2009, Effects of reaction conditions on nuclear laundry water treatment in Fenton process, Journal of Hazardous Materials, Vol. 164, 1468–1473. |
| [109] | S. Navalon, M. Alvaro, H. Garcia, 2010, Heterogeneous Fenton catalysts based on clays, silicas and zeolites, Applied Catalysis B: Environmental, Vol. 99, 1–26. |
| [110] | A.D. Bokare, W. Choi, 2014, Review of Iron-Free Fenton-Like Systems for Activating H2O2 in Advanced Oxidation Processes. In Press, Journal of Hazardous Materials. |
| [111] | P.V. Nidheesh, R. Gandhimathi, 2012, Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination, Vol. 299, 1-15. |
| [112] | Z. Wang, G. Li, Fang Yang, Y. Chen, P. Gao, 2011, Electro-Fenton degradation of cellulose using graphite/PTFE electrodes modified by 2-ethylanthraquinone, Carbohydrate Polymers, Vol. 86 (4), 1807–1813. |
| [113] | A. Babuponnusami, K. Muthukumar, 2012, Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro- Fenton processes, Chemical Engineering Journal, Vol. 183, 1–9. |
| [114] | B. Wang, L. Gu, H. Ma, 2007, Electrochemical oxidation of pulp and paper making wastewater assisted by transition metal modified kaolin, Journal of Hazardous Materials, Vols. 143 (1-2), 198–205. |
| [115] | C.B. Chidambara Raj, Han Li Quen, 2005, Advanced oxidation processes for wastewater treatment: Optimization of UV/H2O2 process through a statistical technique, Chemical Engineering Science, Vol. 60 (9), 5305–5311. |
| [116] | M. Pera-Titus, V. García-Molina, M. A. Baños, 2004, Degradation of chlorophenols by means of advanced oxidation processes: a general review, Applied Catalysis B: Environmental, Vol. 47, 219–256,. |
| [117] | J.B. Rodríguez, A. Mutis, M.C. Yeber, J. Freer, J. Baeza, H.D. Mansilla, 1999, Chemical degradation of EDTA and DTPA in a totally chlorine free (TCF) effluent, Water Science and Technology, Vols. 40 (11-12), 267–272. |
| [118] | N. H Ince, I. G Apikyan. Combination of activated carbon adsorption with light-enhanced chemical oxidation via hydrogen peroxide, Water Research, 2000, Vol. 34 (17), 4169–4176. |
| [119] | P. R. Gogate, A. B. Pandit, 2004, A review of imperative technologies for wastewater treatment II: hybrid methods, Advances in Environmental Research, Vol. 8, 553–597. |
| [120] | T. Rodriguez, D. Botelho, E. Cleto, 2008, Tratamiento de efluentes industriales de naturaleza recalcitrante usando ozono, peróxido de hidrógeno y radiación ultravioleta. Rev. Fac. Ing. Univ. Antioquia.. 24-38, Vol. 46. |
| [121] | R. Alnaizy, A. Akgerman, 2000, Advanced oxidation of phenolic compounds., Advances in Environmental Research, Vol. 4 (3), 233–244. |
| [122] | T. Ratpukdi, S. Siripattanakul, E. Khan, 2010, Mineralization and biodegradability enhancement of natural organic matter by ozoneeVUV in comparison with ozone, VUV, ozoneeUV, and UV: Effects of pH and ozone dose, Water research, Vol. 44, 3531-3543. |
| [123] | I. Oller, S. Malato, J.A. Sánchez-Pérez, 2011, Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review, Science of The Total Environment, Vol. 409 (20), 4141–4166. |
| [124] | D. C. Botía, M. S. Rodríguez, V. M. Sarria, 2012, Evaluation of UV/TiO2 and UV/ZnO photocatalytic systems coupled to a biological process for the treatment of bleaching pulp mill effluent, Chemosphere, Vol. 89 (6), 732–736. |
| [125] | Xiang-Rong Xu, Xiao-Yan Li, Xiang-Zhong Li, Hua-Bin L, 2009, Degradation of melatonin by UV, UV/H2O2, Fe2+/H2O2 and UV/Fe2+/H2O2 processes, Separation and Purification Technology, Vols. 68 (2-5), 261–266. |
| [126] | F. J. Benitez, J. Beltran-Heredia, J. L. Acero, F. J. Rubio, 2000, Contribution of free radicals to chlorophenols decomposition by several advanced oxidation processes, Chemosphere, Vol. 41, 1271-1277. |
| [127] | A.M. Amat, A. Arques, F. López, M.A. Miranda, 2005, Solar photo-catalysis to remove paper mill wastewater pollutants., Solar Energy, Vol. 79 (4), 393–401. |
| [128] | D. Hermosilla, N. Merayo, R. Ordóñez, Á. Blanco, 2012, Optimization of conventional Fenton and ultraviolet-assisted oxidation processes for the treatment of reverse osmosis retentate from a paper mil, Waste Management, Vol. 32 (6), 1236–1243. |
| [129] | M. E.T. Sillanpää, T. A. Kurniawan, W. Lo, 2011, Degradation of chelating agents in aqueous solution using advanced oxidation process (AOP), Chemosphere, Vol. 83 (11), 1443–1460. |
| [130] | S. Esplugas, J. Gim!enez, S Contreras, E. Pascual, 2002, Comparison of different advanced oxidation processes for phenol degradation, Water Research 36, Vol. 36, 1034–1042. |
| [131] | M. S. Lucas, J. A. Peres, C Amor, L Prieto-Rodríguez, M I. Maldonado, S Malato, 2012, Tertiary treatment of pulp mill wastewater by solar photo-Fenton., Journal of Hazardous Materials, Vols. 225–226, 173–18. |
| [132] | T. S. Jamil, M. Y. Ghaly, I. E. El-Seesy, E. R. Souaya, R. A. Nasr, 2011, A comparative study among different photochemical oxidation processes to enhance the biodegradability of paper mill wastewater, Journal of Hazardous Materials, Vol. 185, 353–358. |
| [133] | J. L. Tambosi, M. Di Domenico, R. F. P. M. Moreira. Pre-oxidation and coagulation of paper and pulp wastewater by fenton-like process. Departamento de Engenharia Química e Engenhari Federal de Santa Catarin2nd Mercosur Congress on Chemical Engineering, 4th Mercosur Congress on Process Systems Engineering. |
| [134] | M. Faouzi, P. Cañizares, A. Gadri, J. Lobato, B. Nasr, R. Paz, M.A. Rodrigo, C. Saez, 2006, Advanced oxidation processes for the treatment of wastes polluted with azoic dyes, Electrochimica Acta, Vol. 52 (1), 325–331. |
| [135] | B. Gözmen, B. Kayan, A. Murat Gizir, A. Hesenov, 2009, Oxidative degradations of reactive blue 4 dye by different advanced oxidation methods, Journal of Hazardous Materials, Vol. 168, 129–136. |
| [136] | S.G. Schrank, H.J. Jose, R.F.P.M. Moreira, H.Fr. Schroder, 2005, Applicability of Fenton and H2O2/UV reaction in the treatment of tannery wastewaters, Chemosphere, Vol. 60, 644–655. |
| [137] | H. Zangeneh, A.A.L. Zinatizadeh, M. Feizy. A comparative study on the performance of different advanced oxidation processes (UV/O3/H2O2) treating linear alkyl benzene (LAB) production plant's wastewater. In press. Journal of Industrial and Engineering Chemistry, 2013. |
| [138] | C. Ciotti, R. Baciocchi, T. Tuhkane, 2009, Influence of the operating conditions on highly oxidative radicals generation in Fenton's systems, Journal of Hazardous Materials, Vol. 161 (1), 402–408. |
| [139] | H. Al-Sheeha, M. Marafi, A. Stanislaus, 2008, Reclamation of alumina as boehmite from an alumina-supported spent catalyst. ., Int. J. Miner. Process, Vol. 88, 59–64. |
| [140] | G. Cao, M. Sheng, W. Niu, Y. Fei, D. Li, 2009, Regeneration and reuse of iron catalyst for Fenton-like reactions, Journal of Hazardous Materials, Vol. 172, 1446–1449. |
| [141] | F. Larachi, S. Lévesque, A. Sayari, 1998, Wet oxidation of acetic acid by H2O2 catalyzed by transition metal-exchanged NaY zeolites., J. Chem. Technol. Biotechnol, Vol. 73, 127-130. |
| [142] | P. Cañizares, R. Paz, C. Sáez, M. A. Rodrigo, 2009, Costs of the electrochemical oxidation of wastewaters: A comparison with ozonation and Fenton oxidation processes, Journal of Environmental Management, Vol. 90 (1), 410–420. |
| [143] | T. Coenen, W. Van de Moortel, F. Logist, J. Luyten, J. F.M. Van Impe, J. Degrèv, 2013, Modeling and geometry optimization of photochemical reactors: Single- and multi-lamp reactors for UV–H2O2 AOP systems, Chemical Engineering Science, Vol. 96 (7), 174–189. |
| [144] | X. Liu, X. Sun, D. Li, W. Li, Y. Huang, G. Sheng, H. Yu, 2012, Anodic Fenton process assisted by a microbial fuel cell for enhanced degradation of organic pollutants.., Water Research, Vol. 46 (14), 4371–4378. |
| [145] | M. A. Oturan, N. Oturan, M. C. Edelahi, F. I. Podvorica, K. El Kacemi, 2011, Oxidative degradation of herbicide diuron in aqueous medium by Fenton's reaction based advanced oxidation processes, Chemical Engineering Journal, Vol. 171 (1), 127–135. |
| [146] | E. J. Rosenfeldt, K. G. Linden, S. Canonica, U. Gunten, 2006, Comparison of the efficiency of radical dotOH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2., Water Research, Vol. 40 (20), 3695–3704. |
| [147] | M. S. Lucas, J. A. Peres, G. Li Puma, 2010, Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economic, Separation and Purification Technology, Vol. 72 (3), 235–241. |
| [148] | S. Parra, S. Malato, C. Pulgarín, 2002, New integrates photocatalytic–biological flow system using supported TiO2 and fixed bacteria for the mineralization of isoproturon, Applied Catalysis B: Environmental, Vol. 36, 131–144. |
| [149] | D. R. Manenti, A. Módenes, P. Soares, F. Espinoza-Quiñones, R. Boaventura, R. Bergamasco, V. Vilar. Assessment of a multistage syste based on electrocoagulation, solar photo Fenton and biological oxidation processes for real textile wasterwater. In Press, Chemical Engineering Journal, 2014. |
| [150] | C. Sirtori, A. Zapata, I. Oller, W. Gernjak, A. Aguera, S. Malato, 2009, Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment.., Water Research, Vol. 43, 661-668. |
| [151] | A. Zapata, I. Oller, C. Sirtori, A. Rodríguez, J. Sánchez-Pérez, A. Lopez, M. Mezcua, S. Malato, 2010, Decontamination of industrial wastewater containing pesticides by combining large-scale homogeneous solar photocatalysis and biological treatment., Chemical Engineering Journal, Vol. 160, 447–456. |
| [152] | X.-J. Wang, Y. Song, J.-S. Mai, 2008, Combined Fenton oxidation and aerobic biological processes for treating a surfactant wastewater containing abundant sulfate., Journal of Hazardous Materials, Vol. 160, 344–348. |
| [153] | R. Toor, M. Mohsen, 2007, UV-H2O2 based AOP and its integration with biological activated carbon treatment for DBP reduction in drinking water, Chemosphere, Vol. 66 (11), 2087–2095. |
| [154] | Fahmi, W. Nishijima, M. Okada, 2003, Improvement of DOC removal by multi-stage AOP-biological treatment, Chemosphere, Vol. 50 (8), 1043–1048. |
| [155] | M.M. Ballesteros Martín, J.L. Casas López, I. Oller, S. Malato, J.A. Sánchez Pérez, 2010, A comparative study of different tests for biodegradability enhancement determination during AOP treatment of recalcitrant toxic aqueous solutions, Ecotoxicology and Environmental Safety, Vol. 73 (6), 1189–1195. |
| [156] | S. Malato, P. Fernández-Ibañez, M.I. Maldonado, J. Blanco, W. Gernjak, 2009, Decontamination and disinfection of water by solar photocatalysis: recent overview and trends, Catalysis Today, Vol. 147, 1–59. |
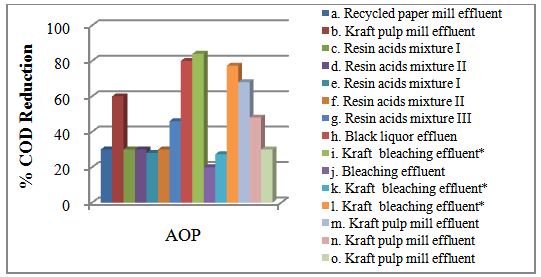
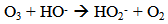
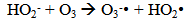
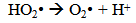
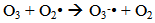
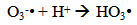
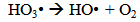
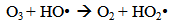
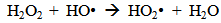
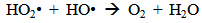
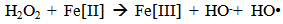
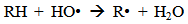
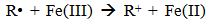
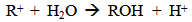
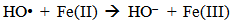
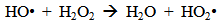
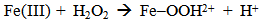
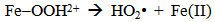
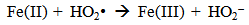
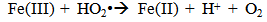
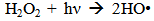
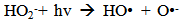
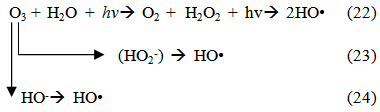 Accordingly, the conditions to be considered for O3/UV processes can be summarized as [119]: high partial pressures and continuous bubbling of ozone; low initial concentration of contaminants (in general, the effluent requires dilution); temperature optimization taking into account the increase in the rate of degradation and the low solubility of ozone at high temperatures; neutral or slightly alkaline pH (range 7-8); minimum presence of radical scavengers because they inhibit the process of degradation. These combined treatments which involve photodegradation are important for removing aromatic hydrocarbons and chlorinated phenolic compounds present in some bleaching effluents of kraft pulp [123]. Bleaching processes using chlorine generate bioaccumulative and carcinogenic substances (such as chlorophenols), which are difficult to remove with a biological treatment or by oxidative treatments applied individually [124]. The efficiency of degradation of Fenton process is also accelerated by irradiation with UV light [125]. It is expected also a improvement of the efficiency in the oxidation reaction, because in this case two different pathways contribute to the generation of free radicals (UV radiation and iron salts) and, therefore, its concentration should be high [126]. Fenton and photo Fenton efficiencies have been monitored in the treatment of an effluent from a TMP pulping process. The increase in discoloration produced by the last one was attributed to the increased radical attack to the double bonds of the lignin in the effluent, responsible for its color [23]. Color removal was also verified when treating bleach effluents from unbleached Kraft pulping processes [127]. Some stages in the pulp and paper mill require recirculating water of high quality. In these cases, reverse osmosis systems are implemented as the final step of the different treatments applied for the reduction of recalcitrant toxic compounds. The photo-Fenton system has been proved to be a viable alternative in these cases requiring strict processing conditions [128]. Fenton oxidation has also been evaluated for oxidation of chelants, reaching reductions of about 90%. The oxidation rate was increased by photo-Fenton treatment [129]. This oxidation treatment is also highly efficient when applied to the effluents of a wastepaper plant (TOC concentration: 332mgL−1 and COD: 1286mgL−1). Generally, over 90% of TOC can be removed with optimized conditions, especially at temperatures about 50°C. Because the temperature of the effluent is normally between 40 and 50ºC, no energy input is required. Therefore, efficient TOC removal is highly possible under the usual operation conditions [53].When testing various oxidizing systems on a solution of phenol (93-105 mgL-1), it was found that no combination of ozone (O3/H2O2, O3/UV and O3/UV/H2O2) enhanced the rate of degradation with respect to the use of ozone alone. Concerning peroxide treatment, the degradation rate of the UV/H2O2 process was almost five-fold higher than with UV. Fenton's reagent resulted in a degradation 40-fold faster than the UV process. The highest percentages of reduction achieved were, O3: 100%; O3/H2O2: 92.5% UV: 24.2%; UV/H2O2: 90.6%; O3/UV/H2O2: 99.4% Fe (II) / H2O2: 100% [130].Various oxidative systems have been evaluated on different streams of wastewaters from pulp and paper mills. Applying different iron doses (1.3, 20, and 50 mgL-1) in the Fenton's reagent on an outgoing effluent from a biological treatment (initial COD: 898,9 mgL-1), COD removal achieved was 4, 18, and 36%, respectively. This low rate of degradation observed was attributed to the poor regeneration of ferrous ions (reduction of Fe(III) to Fe(II)) in the absence of UV light. On the contrary, when photo-Fenton was applied with 5 and 10 mgL-1 of Fe(II), COD removal rates exceeded 90% [131]. The use of hydrogen peroxide alone resulted in too low removal values, also the UV treatment was ineffective by itself, producing unacceptably low color and TOC reductions (initial TOC: 110 mgL-1), UV/H2O2 treatment performance was also unsatisfactory and the same results were verified in the treatment with ozone of organochlorine compounds. Both, Fenton or photo-Fenton treatments can be used for effective removal of color, organochlorine compounds and TOC of those wastewaters, reaching reductions of about 80% in all parameters. However, the photo-Fenton treatment seems to be more advantageous because requires much less time to react and smaller reactor volumes, compared to Fenton treatment [51].The degradation of the organic compounds in the bleaching effluents from a Kraft pulp mill has been successfully carried out by applying photo-Fenton and Fenton treatments. Temperature is a key parameter that significantly increases reaction rates whereas UV irradiation improves TOC removal. At high temperature the system has similar reaction rates in both, Fenton and photo-Fenton. In all experiments, the color of the samples was reduced over 90% at the end of the reaction [61].The effect of the initial concentration of hydrogen peroxide in UV/H2O2, Fenton and Photo-Fenton in the treatment of the effluent from a recycled cardboard mill (COD: 10300 mgL-1) has been reported. Photo-Fenton treatment produced the greatest reduction in COD (76%) compared with UV/H2O2 and Fenton, in 45 minutes [132]. With the same purpose, a papermaking effluent with a COD of 950 mgL-1 was treated by the Fenton method, obtaining color and COD reductions of 95% and 50% respectively [133].
Accordingly, the conditions to be considered for O3/UV processes can be summarized as [119]: high partial pressures and continuous bubbling of ozone; low initial concentration of contaminants (in general, the effluent requires dilution); temperature optimization taking into account the increase in the rate of degradation and the low solubility of ozone at high temperatures; neutral or slightly alkaline pH (range 7-8); minimum presence of radical scavengers because they inhibit the process of degradation. These combined treatments which involve photodegradation are important for removing aromatic hydrocarbons and chlorinated phenolic compounds present in some bleaching effluents of kraft pulp [123]. Bleaching processes using chlorine generate bioaccumulative and carcinogenic substances (such as chlorophenols), which are difficult to remove with a biological treatment or by oxidative treatments applied individually [124]. The efficiency of degradation of Fenton process is also accelerated by irradiation with UV light [125]. It is expected also a improvement of the efficiency in the oxidation reaction, because in this case two different pathways contribute to the generation of free radicals (UV radiation and iron salts) and, therefore, its concentration should be high [126]. Fenton and photo Fenton efficiencies have been monitored in the treatment of an effluent from a TMP pulping process. The increase in discoloration produced by the last one was attributed to the increased radical attack to the double bonds of the lignin in the effluent, responsible for its color [23]. Color removal was also verified when treating bleach effluents from unbleached Kraft pulping processes [127]. Some stages in the pulp and paper mill require recirculating water of high quality. In these cases, reverse osmosis systems are implemented as the final step of the different treatments applied for the reduction of recalcitrant toxic compounds. The photo-Fenton system has been proved to be a viable alternative in these cases requiring strict processing conditions [128]. Fenton oxidation has also been evaluated for oxidation of chelants, reaching reductions of about 90%. The oxidation rate was increased by photo-Fenton treatment [129]. This oxidation treatment is also highly efficient when applied to the effluents of a wastepaper plant (TOC concentration: 332mgL−1 and COD: 1286mgL−1). Generally, over 90% of TOC can be removed with optimized conditions, especially at temperatures about 50°C. Because the temperature of the effluent is normally between 40 and 50ºC, no energy input is required. Therefore, efficient TOC removal is highly possible under the usual operation conditions [53].When testing various oxidizing systems on a solution of phenol (93-105 mgL-1), it was found that no combination of ozone (O3/H2O2, O3/UV and O3/UV/H2O2) enhanced the rate of degradation with respect to the use of ozone alone. Concerning peroxide treatment, the degradation rate of the UV/H2O2 process was almost five-fold higher than with UV. Fenton's reagent resulted in a degradation 40-fold faster than the UV process. The highest percentages of reduction achieved were, O3: 100%; O3/H2O2: 92.5% UV: 24.2%; UV/H2O2: 90.6%; O3/UV/H2O2: 99.4% Fe (II) / H2O2: 100% [130].Various oxidative systems have been evaluated on different streams of wastewaters from pulp and paper mills. Applying different iron doses (1.3, 20, and 50 mgL-1) in the Fenton's reagent on an outgoing effluent from a biological treatment (initial COD: 898,9 mgL-1), COD removal achieved was 4, 18, and 36%, respectively. This low rate of degradation observed was attributed to the poor regeneration of ferrous ions (reduction of Fe(III) to Fe(II)) in the absence of UV light. On the contrary, when photo-Fenton was applied with 5 and 10 mgL-1 of Fe(II), COD removal rates exceeded 90% [131]. The use of hydrogen peroxide alone resulted in too low removal values, also the UV treatment was ineffective by itself, producing unacceptably low color and TOC reductions (initial TOC: 110 mgL-1), UV/H2O2 treatment performance was also unsatisfactory and the same results were verified in the treatment with ozone of organochlorine compounds. Both, Fenton or photo-Fenton treatments can be used for effective removal of color, organochlorine compounds and TOC of those wastewaters, reaching reductions of about 80% in all parameters. However, the photo-Fenton treatment seems to be more advantageous because requires much less time to react and smaller reactor volumes, compared to Fenton treatment [51].The degradation of the organic compounds in the bleaching effluents from a Kraft pulp mill has been successfully carried out by applying photo-Fenton and Fenton treatments. Temperature is a key parameter that significantly increases reaction rates whereas UV irradiation improves TOC removal. At high temperature the system has similar reaction rates in both, Fenton and photo-Fenton. In all experiments, the color of the samples was reduced over 90% at the end of the reaction [61].The effect of the initial concentration of hydrogen peroxide in UV/H2O2, Fenton and Photo-Fenton in the treatment of the effluent from a recycled cardboard mill (COD: 10300 mgL-1) has been reported. Photo-Fenton treatment produced the greatest reduction in COD (76%) compared with UV/H2O2 and Fenton, in 45 minutes [132]. With the same purpose, a papermaking effluent with a COD of 950 mgL-1 was treated by the Fenton method, obtaining color and COD reductions of 95% and 50% respectively [133]. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML