-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2014; 4(1): 11-17
doi:10.5923/j.ajee.20140401.03
Kinetic Study of Adsorption Rhodamine 6G Dye from Aqueous Solutions Using Bentonite Clay
Ahlam M. Farhan , Anfal S. Sameen
Department of chemistry, college science for women, University of Baghdad, Baghdad, Iraq
Correspondence to: Ahlam M. Farhan , Department of chemistry, college science for women, University of Baghdad, Baghdad, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
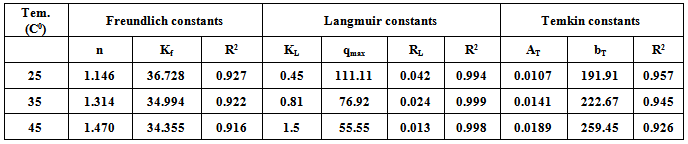
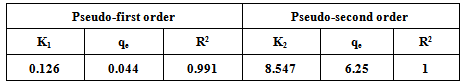
The kinetics of adsorption of Rh6G on bentonite has been investigated in a batch adsorption process. The adsorption of Rh6G was found to be dependent on pH, initial concentration, adsorbent dose, contact time and Temperature. The result indicated that, optimum contact time was 30 minutes and the adsorbent dose was 0.2g of bentonite. The Langmuir, freundlich and Temkin isotherm models were analyzed for the equilibrium adsorption data and the various isotherm parameters were evaluated. The Rh6G on bentonite follows pseudo-second order rate kinetics.
Keywords: Rh6G, Bentonite, Kinetics model adsorption
Cite this paper: Ahlam M. Farhan , Anfal S. Sameen , Kinetic Study of Adsorption Rhodamine 6G Dye from Aqueous Solutions Using Bentonite Clay, American Journal of Environmental Engineering, Vol. 4 No. 1, 2014, pp. 11-17. doi: 10.5923/j.ajee.20140401.03.
Article Outline
1. Introduction
- Dyes are widely used in industries such as textiles, paints, rubber plastic, paper, cosmetics, etc.. The presence of dyes is most often in industrial processes and their removal may be an environmental problem[1-4]. The treatment of dyes in industrial wastewater possesses several problems since dyes are generally difficult to photodegrade and biodegradation[5]. Research is being carried out for removal dyes using chemical, physicochemical and biological treatment technologies, such as cloud point extraction, oxidation processes, coagulation flocculation, and adsorption have been used for the removal of Colored dyes from wastewater[6-9]. However, adsorption is considered more effective and less expensive them other technologies[10-12]. In the present study, detailed are carried out to remove this dye by adsorption technique using bentonite as an adsorbent. Various parameters affecting adsorption process, such as contact time, initial dye concentration, temperature and PH were investigated. In addition, kinetic parameters were also calculated to determine adsorption mechanism was fitted into adsorption isotherms in order to give the best fit correlation.
2. Materials and Methods
2.1. Preparation of Adsorbed and Characterization
- The bentonite clay was supplied from the state company for geological survey and mining – Iraq. The bentonite had the following composition with particle size less than 75 µm.
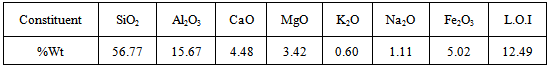 The molecular formula of bentonite could be written as:Mg2Al10Si24O60(OH)12[Na,Ca]The ground adsorbent was washed thoroughly with distilled water in order to remove any dissolved impurities and then dried at 160℃ and sorted for further use.
The molecular formula of bentonite could be written as:Mg2Al10Si24O60(OH)12[Na,Ca]The ground adsorbent was washed thoroughly with distilled water in order to remove any dissolved impurities and then dried at 160℃ and sorted for further use.2.2. Adsorbate
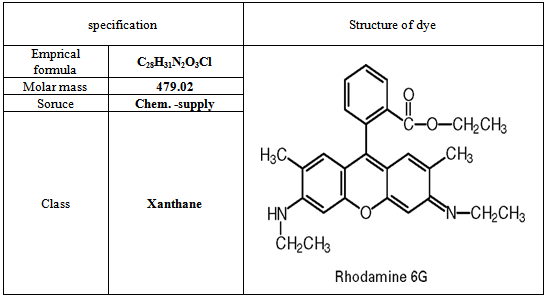
- The dye used in this work was Rhodamine 6G, it was choice in this study because it has a strong adsorption onto solids and also for its high solubility in water. The characteristics and chemical structure of this dye are listed in Table 1. A stock solution of Rhodamine 6G was prepared by dissolving its 1.0 gm in 1000 mL distilled water. The stock solution was diluted accordingly to obtain fresh solutions of desired concentrations. To determine the dye concentration, a calibration curve was first obtained from a series of predetermined concentration of dye solution Fig.1. The maximum absorbance of the dye was confirmed by scanning the dye solution over the spectral range of 400-600nm by using UV-VIS spectrophotometer (UV-Visible spectrophotometer, Double beam, Shimadzu. PC 1650, Japan). The absorbance of those standard samples was measured at the corresponding maximum wavelength (λ max=524nm).
|
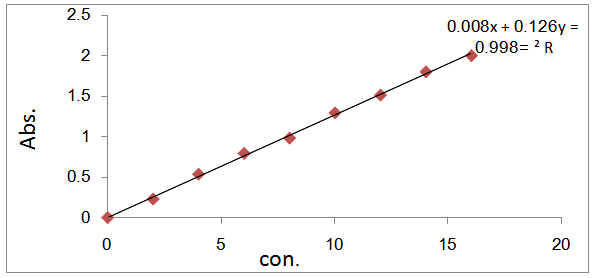 | Figure 1. calibration curve of Rhodamine 6G dye at (λ max=524) |
2.3. Batch Adsorption Study
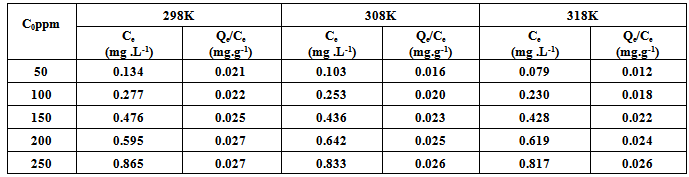
- The adsorption isotherm have been determined by Rhodamine 6G dye solution of known, initial concentration to be mixed with accurately weight amount of bentonite in tightly closed flask at certain temperature and pH.The amount of bentonite has been 0.2 gm/50ml solution. A constant temperature and pH were achieved using a shaker water bath (JEIOTECH BS-1). The flask s were subsequently capped and placed on controlled temperature mechanical shaker at a speed of 160 rpm at desired temperature and equilibrated for 30 min .After adsorption, the solution was separated by centrifuge at 3000 rpm for 15 min and super solution was subjected to analysis using UV-VIS technique at wavelength of maximum absorbance (524 nm), the absorbance is then converted to concentration using the calibration curve. The same experiment was repeated at different initial concentration temperature and pH.The amount of adsorbed Rh6G at any time, qt (mg/g) was calculated using Eq.1
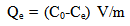 | (1) |
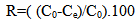 | (2) |
2.4. Effect of Adsorption Parameter
- To study the effect of temperature, adsorption experiments were conducted at 25, 35 and 45℃, respectively. The influence of the pH was investigated at values: 3 ,6 ,9 and 12. Rhodamine 6G solution pH was Adjusted using 0.1 M NaOH and 0.1 M HCl solution and measured using (pH-Mete r-Hanna-HI-8417 / England). Adsorbent adsorbate dosage used in this study was in the range 0.2gm to 1gm.
3. Result and Discussion
3.1. Effect of Contact Time
- Adsorption study was carried out by adding a known amount of bentonite (0.2gm), 50ppm of Rhodamine6G dye solution was examined at different time as shown in Fig.2. The result showed that the rate of adsorbed Rhodamine6G onto natural bentonite was rapid and then became slower near the equilibrium. Most of the maximum quantity adsorption of Rh 6G dye was attained after about 30min of shaking time at different initial concentration. The results indicated that the fact that a large number of vacant surface site were available for adsorption during the initial stage. Near the equilibrium the remaining vacant surface sites were difficult to be occupied due to the slow pore diffusion of the solute molecules on the solid and the bulk phase.
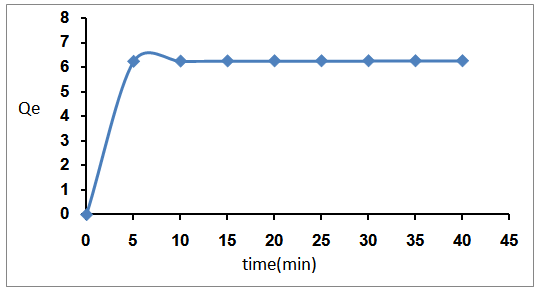 | Figure 2. Effect of shaking Time on removal efficiency |
3.2. Effect of pH
- The pH has been considered an important parameter in adsorption studies it controls the adsorption at the adsorbent solution interface Experiments were conducted varying the solution pH from 3to 12 while the remain of the factors were kept constant. The maximum removal of Rh6G was observed at pH = 6. The adsorption mechanism on the bentonite reflect the nature of physiochemical interaction of the solution.
3.3. Effect of Adsorbent Dose
- The effect of adsorbent dose is an important parameter because this determine the capacity of adsorption for aginen initial concentration. The adsorption efficiency of R6G as a function of adsorbent dose was studied. The percentage of the R6G adsorption steeply increases with the adsorbent dose loading up to (0.2gm - 1gm ). This result can be explained by the fact that the adsorption sites remain unsaturated during the adsorption reaction where as the number of a sites available for adsorption site increases by increasing the adsorbent dose. The maximum adsorption (99%) was attained at adsorbent dose 0.2gm. Therefore, the optimum adsorption dose was taken as 0.2gm forfurthar experiments.
3.4. Adsorption Isotherms
- The adsorption mechanism and relationship between the concentration of the adsorbate and adsorption capacity of both the adsorbent was studied using different adsorption isotherm.
3.4.1. Langmuir Adsorption Isotherm
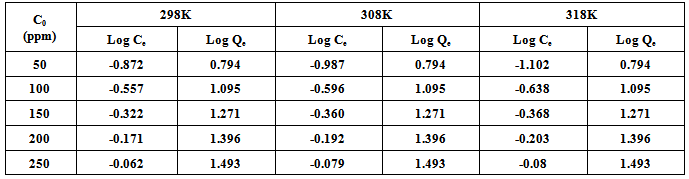
- The Langmuir isotherm describes the formation of a monolayer adsorbate on the uniform surface of the adsorbent, and after that no further adsorption takes place. As such the surface will eventually reach a saturation point where the maximum adsorption of the surface will be achieved. The model assumes uniform energies of adsorption on to the surface and no transmigration of the adsorbate in the plane of the surface[14].The linear form of the Langmuir isotherm model is described as:
 | (3) |
|
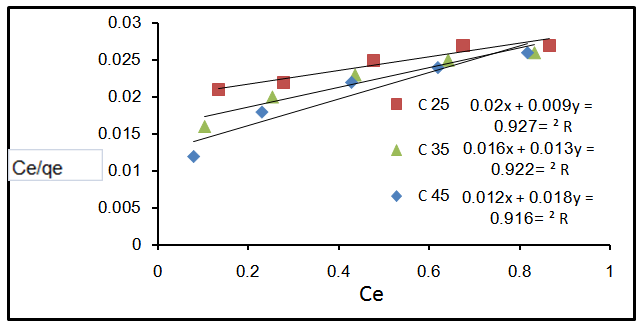 | Figure 3. Effect of Langmuir equation at different temperature |
|
|
3.4.2. Freundlich Isotherm
- This is commonly applies to adsorption on heterogeneous surfaces. These data often fit the empirical equation proposed by Freundlich[16].
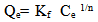 | (4) |
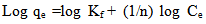 | (5) |
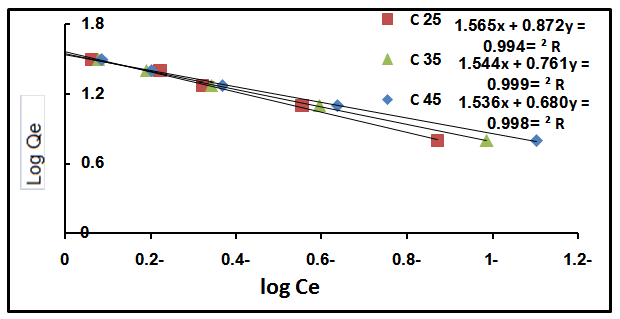 | Figure 4. Effect of Freundlich equation at different temperature |
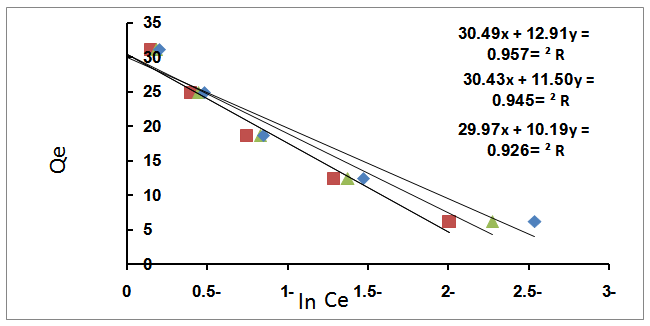 | Figure 5. Effect of Temkin equation at different temperature |
|
3.4.3. Temkin Isotherm
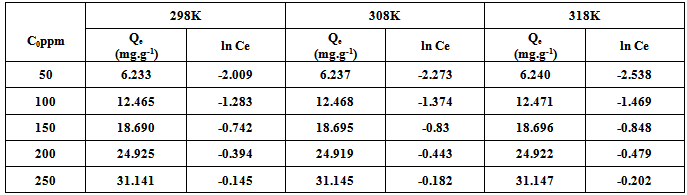
- This isotherm is used to describe the adsorption characteristics, the model assumes that heat of adsorption of all molecules in the large decreases linearly with coverage due to adsorbent –adsorbate interactions and that the adsorption is characterized by a uniform distribution of the binding energies up to some maximum binding energy was carried out by plotting qe against ln Ce and the constant were determined from the slope and intercept[15].
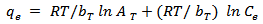 | (6) |
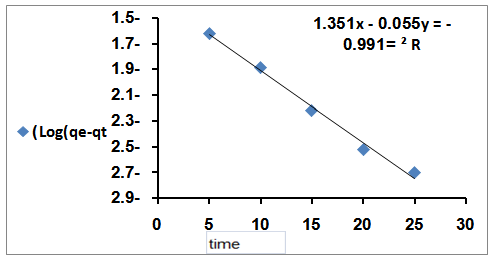 | Figure 6. The applicability of the first order kinetic model to Rh6G on bentonite clay |
3.5. Adsorption Kinetics
- Constant from two kinetic models , pseudo first-order and pseudo second-order were fit to experimental data to examine the adsorption kinetics of R6G by bentonite.
3.5.1. Pseudo First-order Model
- The pseudo-first order equation of lagergren is given by equation.
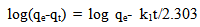 | (7) |
|
3.5.2. Pseudo-second Order Model
- The pseudo second-order model, which has been applied for analyzing adsorption kinetics rate is given by equation :-
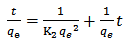 | (8) |
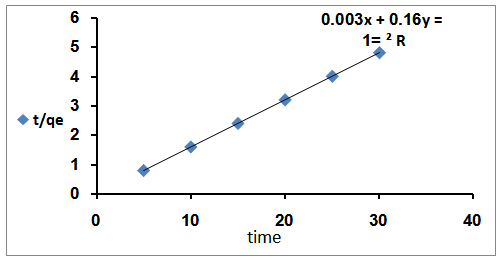 | Figure 7. t/q against time of adsorption of Rh6G on to bentonite clay |
4. Conclusions
- In present study bentonite clay was selected as alocal, cheap and readily available adsorbent for the removal of Rh6G from aqueous solution. bentonite was studied by batch adsorption experiments. The adsorption of Rh6G was found to be dependent, pH, Initial concentration, adsorbent dose, contact time and temperature. The Langmuir, Freundlich and Temkin isotherm models were analyzed, adsorption data and the various isotherm parameters were evaluated. The Rh6G on bentonite follows pseudo-second-order rate kinetics.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML