| [1] | Gupta, V.K., Ali, I., (2000). Utilization of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep.Purif.Technol.18, 131-140. |
| [2] | Fenglian, Fu, Wang, Qi, (2011). Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 92 (3), 407-418. |
| [3] | Yang, H., Rose, N. L., (2003). Distribution of Hg in the lake sediments across the UK. Sci. Tot. Environ., 304, 391-404. |
| [4] | Sanders, T., Liu, Y., Buchner, V., Tchounwou, P.B., (2009). Neurotoxic effects and biomarkers of lead exposure: a review, Rev Environ Health, 24(1), 15-45. |
| [5] | Sax, N.I., Lewis, R.J., (2004). Sax’s dangerous properties of industrial materials, 11th ed. Hoboken New Jersey, John Wiley. |
| [6] | Kadir, M.M., (2008). Status of children’s blood lead levels in Pakistan: implications for research and policy. Public Health, 122, 708-715. |
| [7] | Rahbar, M.H., (2003). Factors associated with elevated blood lead concentration in children in Karachi, Pakistan. Bulletin of the World Health Organization, 2002, 80,769-775. |
| [8] | Phan, N. H., Rio, S., Faur, C., LeCoq, L., Le Cloirec, P., and Nguyen, T. H., (2006).Production of fibrous activated carbons from natural cellulose (jute, coconut) fibers for water treatment applications, Carbon 44(12), 2567-2577. |
| [9] | Chavez-Guerrero, L., Rangel-Mandez, R., Munoz-Sandoval, E., Cullen, D.A., Smith, D. J., Terrones, H., Terrones, M., (2008). Production and detail characterization of bean husk-based carbon: Efficient cadmium(II) removal from aqueous solutions, water Res. 42(13), 3473-3479. |
| [10] | El-Hendawy, A. N. A., (2003). Influence of HNO3 oxidation on the structur and adsorption properties of corncob-based activated carbon, Carbon 41(4), 713-722. |
| [11] | Shibi, I. G., Anirudhan, T. S., (2006). Polymer-grafted banana (Musa paradisiaca) stalk as an adsorbent for removal of lead(II) and cadmium(II) ions from aqueous solutions: Kinetic and equilibrium studies, Journal of Chemical Technology Biotechnology 81(3), 4133-444. |
| [12] | Laufenberg G., Kunz B. and Nystroem M., 2003, Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations, Bioresource Technol. 87, 167–198. |
| [13] | Murugesan G.S., Sathishkumar M., Swaminathan K., 2006, Arsenic removal from groundwater by pretreated waste tea fungal biomass, Bioresour. Technol., 97, 483–487. |
| [14] | Hameed B.H., 2009, Spent tea leaves: A new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions, J. Hazard. Mat, 161, 753–759. |
| [15] | Wasewar K.L., Atif M., Prasad B., Mishra I.M., 2009, Batch adsorption of zinc on tea factory waste, Desalination, 244, 66–71. |
| [16] | Boehm HP (2008). Surface chemical characterization of carbons from adsorption studies. In Adsorption by carbons; Elsevier: Amsterdam, 301-27. |
| [17] | Moreno-Castilla, C., Carrasco-Marin, F., Mueden, A., (1997). The Creation of Acid Carbon Surfaces by Treatment with (NH4)2S2O8, Carbon 35, 1619–1626. |
| [18] | Aliya Fazal and Uzaira Rafique (2012). Biosorption of Cadmium on Spent Tea: Green Chemistry Approach. Journal of Water Sustainability, 2(4), 2012, 259–270. |
| [19] | Laura Ldpez, M., Gardea-Torresdey, Peralta-Vldea, J.R., de la Rosa, G., Arrnendiriz, V., Herrera ,I., Troiani, H., Henning, J., (2005). Gold binding by native and chemically modified Hops biomasses, Bioinorganic Chemistry and Applications, 3(1-2), 29-41. |
| [20] | Paul, A., Joseph, K., Thomas, S., (1997).Compos. Sci. Tech. 57, 67. |
| [21] | Sudhir Dahiya, Tripathi, R.M., Hegde, A.G., (2008). Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca shell biomass. Journal of Hazardous Materials, 150, 376-386. |
| [22] | Yunus Pamukoglu, M., Fikret Kargi, (2008). Biological Treatment of Cu(II) Containing Synthetic Wastewater in an Activated Sludge Unit: Copper(II) Ion Toxicity. Environmental Engineering Science, 25(8), 1159-1166. |
| [23] | Tunali, S., Akar, T., O¨ zcan, S.A., Kiran, I., O¨ zcan, A., (2006). Equilibrium and kinetics of biosorption of lead (II) from aqueous solutions by Cephalosporium aphidicola. Sep. Purif. Technol. 47, 105-112. |
| [24] | Nagendra, C.R., Iyengar, L., Venkobachar, C., (1989). Sorption of copper (II) from aqueous phase by waste biomass. Journal of Environmental Engineering,119, 369-377. |
| [25] | Michael Horsfall Jnr., Ayebaemi I. Spiff, (2004). Studies on the effect of pH on the sorption of Pb2+ and Cd2+ ions from aqueous solutions by Caladium bicolor (Wild Cocoyam) biomass, Electronic Journal of Biotechnology ,7(03). |
| [26] | Delgado, A., Anselmo, A.M., Novais, J.M., (1998). Heavy metal biosorption by dried powdered mycelium of Fusarium flocciferum. Water Environment Research, 70, 370-375. |
| [27] | Sag, Y., Ozer, D., Kutsal, T., (1995).A comparative study of the biosorption of lead (II) ions to Z. ramigera and R. arrhizus. Process Biochem., 30, 169-174. |
| [28] | Davis, T.A., Volesky, B., Mucci, A., (2003). A review of the biochemistry of heavy metal biosorption by brown algae. Water Res., 37, 4311-4330. |
| [29] | Senthil Kumar P., Ramakrishnan K., Dinesh Kirupha S., Sivanesan S., (2010). Thermodynamic and kinetic studies of cadmium adsorption from aqueous solution onto rice husk. Brazilian Journal of Chemical Engineering, 27(02), 347 - 355. |
| [30] | Ramírez, E.R., Norma, L., Gutiérrez Ortega, Cesar, A., Contreras Soto, Maria, T., Olguín Gutiérrez, (2009). Adsorption isotherm studies of chromium (VI) from aqueous solutions using sol–gel hydrotalcite-like compounds. J. Hazard. Mater., 172, 1527–1531. |
| [31] | Hall, K.R., Eagleton, L.C., Acrivos, A. and Vermeulen, T., (1996). Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Industrial and Engineering Chemistry. Fundamentals, 5, 212-223. |
| [32] | Sanchez, A.G., Ayuso, E.A., De Blas, O.J., (1999). Sorption of heavy metals from industrial waste water by low-cost mineral silicates. Clay Miner. 34, 469–477. |
| [33] | Wasewar, K.L., Mohammad, A., Prasad, B., Mishra, I.M., (2008). Adsorption of Zn using factory tea waste: kinetics, equilibrium and thermodynamics. CLEAN: Soil, Water, Air, 36(3), 320-329. |
| [34] | Gubbuk, I.H., Gup, R., Kara, H., Ersoz, M., (2009). Adsorption of Cu(II) onto silica gel-immobilized Schiff base derivative. Desalination, 249, 1243–1248. |
| [35] | Tang, X., Li, Z., Chen, Y., (2009). Adsorption behavior of Zn (II) on Chinese loess. J. Hazard. Mater., 161, 824-834. |
| [36] | Onyango, M.S., Kojima, Y., Aoyi, O., Bernardo, E.C., Matsuda, H., 2004. Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J. Colloid Interface Sci., 279, 341-350. |
| [37] | Jude, C. Igwe, Augustin, A. Abia, (2007). Equilibrium sorption isotherm studies of Cd(II), Pb(II) and Zn (II) ions detoxification from waste water using unmodified and EDTA-modified maize husk. Electron. J. Biotechnol., 10 (04). Available from internet:http://www.ejbiotechnology.info/content/vol10/issue4/full/15/index.html. |
| [38] | Mataka, L. M., Sajidu, S.M.I., Masamba, W.R.L., Mwatseteza, J.F.,(2010). Cadmium sorption by Moringa stenopetala and Moringa oleifera seed powders: Batch, time, temperature, pH and adsorption isotherm studies, International Journal of Water Resources and Environmental Engineering, 2(3), 50-59. |
| [39] | Rao, K.R.A., Khan, M.A., (2009). Biosorption of bivalent metal ions from aqueous solution by an agricultural waste: kinetics, thermodynamics and environmental effects. Colloid Surf. A, 332, 121–128. |
| [40] | Anirudhan, T.S., Rijidh, S., (2009). Glutaraldehyede cross-linked epoxyaminated chitosan as an adsorbent for the removal and recovery of copper (II) from aqueous media. Colloid Surf., A, 351, 52-59. |
| [41] | Horsfall, M., Spiff, A.I., (2005). Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium biocolor (wild cocoyam) biomass. Elect. J. Biotechnol., 8, 162-169. |
| [42] | Sudha Bai, R., Emilia Abraham, T., (2002). Studies on enhancement of Cr (VI) biosorption by chemically modified biomass of Rhizopus nigricans, Water ResearchVolume, 36(05), 1224-1236. |
| [43] | Sun, X.F, Sun, R.C, Sun, J.X, (2004). Acetylation of sugarcane bagasse using NBS as a catalyst under mild reaction conditions for the production of oil sorption-active materials. Bioresource Technology, 95(03), 343-350. |

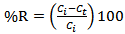
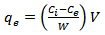
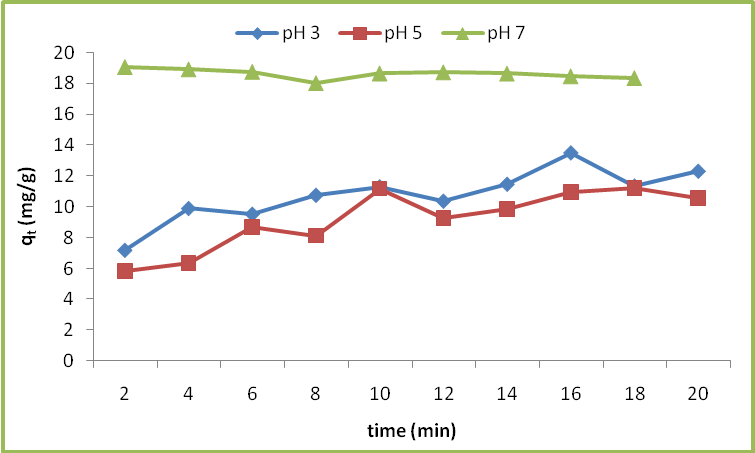
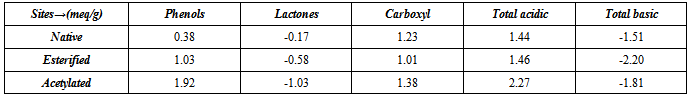
 is less, resulting in lower sorption capacity.
is less, resulting in lower sorption capacity.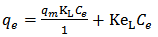
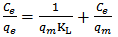
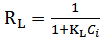
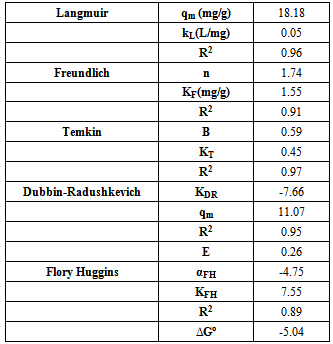
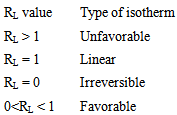 Calculated separation factor was 0.28, proposing favorable sorption of lead upon spent tea at room temperature.
Calculated separation factor was 0.28, proposing favorable sorption of lead upon spent tea at room temperature.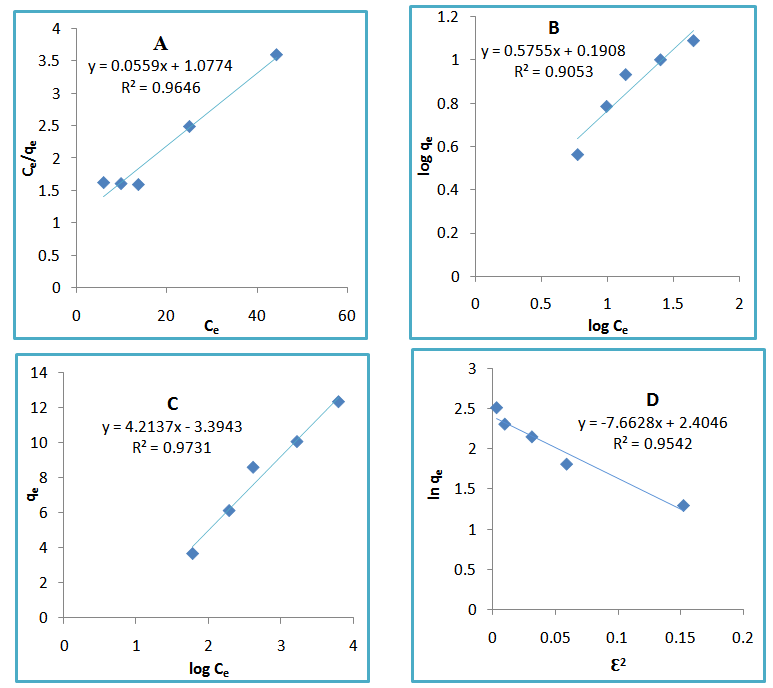
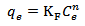
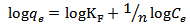
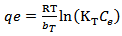
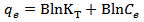
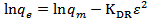
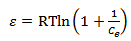
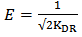
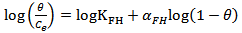
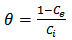
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML