-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2013; 3(4): 195-198
doi:10.5923/j.ajee.20130304.05
Treating Domestic Greywater and Expectations to be Reused
J. S. Almeida, N. R. A. F Rocha, M. R. Franco Junior
FEQUI, Chemical Eng. Faculty, UFU/ Federal University at Uberlândia, Campus Santa Mônica, CEP 38 408 -100, Uberlândia MG Brasil
Correspondence to: M. R. Franco Junior, FEQUI, Chemical Eng. Faculty, UFU/ Federal University at Uberlândia, Campus Santa Mônica, CEP 38 408 -100, Uberlândia MG Brasil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A growing implementation of greywater reuse (GWR) practices may lead to benefits on the wastewater side of the urban water cycle. In addition to the positive effect in decreasing urban water demand, GWR changes the quantity and quality characteristics of domestic wastewater released to sewers and conveyed to wastewater treatment plants. Coagulant selection is not an easy task because one coagulant can remove efficiently the suspended solids but at the same time increase the conductivity. This makes the final selection of coagulants very dependent on the relative importance assigned to each measured parameter. In this work, experiments were carried out using wastewater composed by domestic water, powder soap and kitchen oil in similar quantities of water employed for washing dishes. Measurements of pH, electrical conductivity, temperature, total solids and dissolved oxygen were done by analyzing a sample of 50 mL from the final suspension. It was used a traditional method of coagulation and decanting treatment using some different volume of solutions such as aluminium sulphate (concentration of 1000 and 2000 mg/L), Fe II sulphate and aluminium tripoliphosfate with concentration of 2000 mg/L. According to the findings it could be observed that aluminium sulphate provided good results, reducing total solids and not causing changes in the other parameters, for example, results of pH and dissolved oxygen for the treated water kept closed to the tap water.
Keywords: Greywater, Precipitation, Coagulation, Domestic Water, Treatment
Cite this paper: J. S. Almeida, N. R. A. F Rocha, M. R. Franco Junior, Treating Domestic Greywater and Expectations to be Reused, American Journal of Environmental Engineering, Vol. 3 No. 4, 2013, pp. 195-198. doi: 10.5923/j.ajee.20130304.05.
Article Outline
1. Introduction
- Greywater is water that has been used for washing dishes, laundering clothes, or bathing. Essentially, any water, other than toilet wastes, draining from a household is greywater. In general, this kind of water may contain grease, food particles, hair, and any number of other impurities. Greywater is biologically polluted effluent and its use implies a sanitary risk associated with a potential spread of microorganisms. However, it may still be suitable for reuse.Reusing greywater is appropriate for two purposes: it reduces the amount of freshwater needed to supply a household, and reduces the amount of waste water entering sewer or septic systems. Treated greywater is used for different activities, with toilet flushing and ornamental irrigation two growing applications. Such reuse improves water usage efficiency, and could play a significant role in future water management strategies. However, adequate treatment is required before reuse, and also potential environmental impact cannot be neglected[1]. Investing time and equipment in a system designed to filter, store, and possibly disinfect greywater may make water reuse a more convenient practice. Consequently, there is an evident interest on greywater treatments for successful recycling[2]. Greywater is biologically polluted effluent and its reuse implies a sanitary risk associated with a potential spread of microorganisms. Therefore, disinfection is a key step in any treatment for safe reuse. Chlorinated disinfection agents show high efficacy for disinfection and subsequently, recommendations for more efficient treatments (as coagulation–chlorination and additional removal of organic residuals) have been published[3,4]. Moreover, removal of chemicals (such as parabens) as a consequence of the chlorination process has been also published[5]. Other disinfection strategies such as UV irradiation also provide satisfactory results[6,7].Donner et al. (2010)[8] have reported initial investigations into the fate of a range of pollutants within greywater treatment and reuse systems. However, given the increasing implementation of greywater recycling technology, it is evident that additional research to elucidate the behaviour of xenobiotic micropollutants during greywater treatment would be beneficial. It would also be useful to understand the potential implications of more widespread greywater recycling for urban wastewater loads and dynamics. Greywater treatment and reuse is a very diverse field, encompassing a wide range of potential treatment trains and spatial scales, as well as numerous reuse options[7,9]. Current treatment options vary widely in sophistication from simple filter systems to constructed wetlands, multi-stage biological treatment systems, and membrane bioreactors. Nevertheless, all systems are based on a combination of chemical, physical and biological processes such as adsorption, coagulation, precipitation, filtration, aeration, biodegradation, and disinfection[10].Fatta-Kassinos et al. (2010)[11] have recently reviewed the practice of wastewater reuse for irrigation purposes and concluded that the benefits associated with improved water balances and nutritional levels need to be assessed against the current lack of knowledge relating to possible impacts on ecosystems and human health of the applied organic xenobiotics and heavy metals.Thus, this paper presents an experimental system for kitchen greywater treatment based on coagulation- precipitation. The main objectives are to characterize the collected kitchen greywater, and also to evaluate the efficiency of the treatment system in relation to a set of quality parameters: pH, conductivity, total solids (TS), temperature and dissolved oxygen. This study also intends to discuss the potential for treated greywater to be reused for non-potable water supply, which can represent a significant reduction on potable water consumption in buildings gaining all or part of the referred environmental and economic benefits.
2. Materials and Methods
2.1. Experimental System
- The experimental system of greywater treatment includes a collection tank, a thermostatic bath device (Nova Ética, ± 0.1K) and magnetic stirrer (Marconi TE 085 1700 rpm) that composed a coagulation-precipitation system. The system was built to provide short residence time for the kitchen greywater and with a control of temperature. The glass collection tank is 10 liters. The water entrance is through the top of the tank and the mixing device installed minimizes accumulation of solids. All the solutions used for treatment were prepared using chemicals (aluminium sulphate, Fe II sulphate, sodium carbonate and aluminium tripoliphosfate) supplied by Vetec (Rio de Janeiro- Brasil) with purities higher than 98%. Prepared at room temperature, synthetic greywater was composed by soap (0.0683% in mass), kitchen oil (0.581% in mass) and tap water.
2.2. Treatment with Coagulants
- Chemical oxygen demand (COD) in greywater has three fractions: dissolved, colloidal and suspended. It has been reported that the suspended and colloidal portions represents the major fractions of the total COD of greywater[10-12]. Results from Vasic et al., 2013 has shown that after microfiltration chemical oxygen demand (COD) reduction of 35% while the combined use of natural coagulants and microfiltration achieved 50% of COD reduction compared to the initial value. Therefore, the addition of coagulant agents to remove colloidal and suspended matter and their effect on final greywater can be studied. Since Al(III) and Fe(III) salts are extensively used for this purpose, aluminium sulphate, Fe (II) sulfate and aluminium tripoliphosfate were selected to carry out this study. An adequate amount of coagulant solution was added to 400 mL of greywater and the mixture was stirred magnetically during 12 min. The mixture was then allowed to stand until a clear supernatant (normally from 20 to 30 min). Of this supernant, 50 mL was taken to carry out the analysis of the following parameters: pH (pH meter-Gehaka model PG 1400 ± 0.01), conductivity (λ) (conductivimeter CD 850, ± 0.1µS), temperature (T) (Labortherm, ± 0.1K), dissolved oxygen (Oximeter model DO 5519 ± 0.1 mg/L) and total solids (TS) (oven model 404-3D). For this kind of greywater, no chlorination process has done.A reasonable clarification was observed due to this treatment. Nevertheless, curves were not strongly dependent on either the nature of the coagulant or the added concentration. Sometimes the presence of larger amounts of coagulant agents did not change the parameter as well. Also, it is not possible to know that after the treatment, solution had heavy metals or pollutants inside. In each sample of greywater (A1, A2, A3 e A4) was added a volume of coagulant (C= 2000 mg/L) and the alkalinizing in the same proportion: (A1) 20mL, (A2) 40mL, (A3) 60mL e (A4) 80mL, resulting in the final coagulant concentration of: 80, 160, 240 and 320 mg/L, respectively. After stirring and decanting, three samples, around 40 mL, were withdrawn from each tested greywater by introducing a glass syringe in the center of the vase. Apart from total solids (TS), all parameters were determined using each specific calibrated equipment. One sample was put in the oven at 80℃ for 12 h to obtain total solids. To prevent measurements errors, equipment sensors were washed using distilled water and dried for each procedure done.
3. Results and Discussion
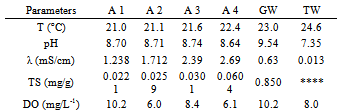
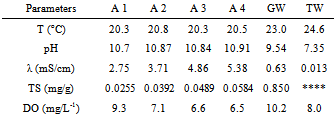
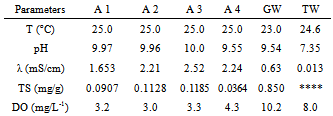
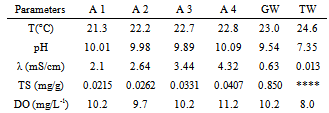
- The results are presented in Tables 1-4. It was used symbols to identify the samples (A1 – A4) and for greywater without treatment (GW). Also, the analysis of tap (or public) water (TW) provided by the city government was added to table for comparisons. Halalsheh et al., 2008 have published data from other researchers for average concentrations of different chemical and biological parameters found in grey water. For example, grey water in their study area is characterized by very high concentrations of TS with average values of 0.845 mg/g. Also, they informed that the pH of grey water varies from 5.58 and 7.60, conductivity is in the range of 1.066-2.398 mS/cm and TS is between 0.168-1.679 mg/g.
|
|
|
|
4. Conclusions
- A water clarification was observed due to this treatment. Nevertheless, decay curves were not strongly dependent on either the nature of the coagulant or the added concentration. The reuse of greywater for non-potable purposes, treatments with coagulants, also make a remarkable improvement in water quality parameters such as dissolved oxygen and total solids. In spite of that, the findings would be completed if other pollutants such as heavy metals had been investigated. From the experimental data, both concentrations of aluminium sulphate coagulants studied have given similar results. Also, as observed by other researchers the precipitated solid phase was brown when using Fe(II) and white for Al(III).Finally, solids, especially total ones, had the highest reduction after treatment however it is believed that total solids could be more affected with a reduction if more decanting time was used.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML