-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2013; 3(4): 187-194
doi:10.5923/j.ajee.20130304.04
Removing General Organic Compounds from Small Quantities of River Water
Daniela Garcia Boneberg, Paulo Sérgio Pimenta, Bruna dos Santos Revolta, Moilton Ribeiro Franco Júnior
Federal University at Uberlândia – Chemical Engineering Faculty Av. João Naves de Ávila, 2121, Bloco 1K do Campus Santa Mônica Uberlândia-MG/Brasil
Correspondence to: Moilton Ribeiro Franco Júnior, Federal University at Uberlândia – Chemical Engineering Faculty Av. João Naves de Ávila, 2121, Bloco 1K do Campus Santa Mônica Uberlândia-MG/Brasil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The properties and amount of NOM (natural organic matter) can hardly affect the process efficiency and it is known that raw water total organic carbon (TOC) levels have a potential to produce concentrations of trihalomethanes (THM). In order to improve and optimize these processes, the characterization and quantification of NOM at different purification and treatment processes stages is important. In this work, experimental measurements were conducted to reduce the concentration of general organic compounds in the natural organic matter (NOM) existing in small amounts of Uberabinha River water in Brazil. Treatment was tested for its efficacy to reduce raw water NOM using gravity filtration with recycled granular activated carbon (RGAC) adsorption. Samples of filtered were collected in intervals of one hour and were analyzed for conductivity, pH and UV254 absorbance. It was verified that pH and conductivity of the final product have not changed comparing to the raw water initial values. Also, the influence of pressure and the carbon particle size was clearly observed in the quantity and quality of the filtered. In a nutshell, RGAC was capable of reducing organic compounds concentration to an acceptable level encouraging its recycling and use.
Keywords: Organic Compounds, Recycled Carbon, Filtration, DOC
Cite this paper: Daniela Garcia Boneberg, Paulo Sérgio Pimenta, Bruna dos Santos Revolta, Moilton Ribeiro Franco Júnior, Removing General Organic Compounds from Small Quantities of River Water, American Journal of Environmental Engineering, Vol. 3 No. 4, 2013, pp. 187-194. doi: 10.5923/j.ajee.20130304.04.
Article Outline
1. Introduction
- Natural organic matter (NOM) consists of a complex mixture composed of different compounds, from largely aliphatic to highly colored aromatics. Some of this organic matter is negatively charged consisting of a wide variety of chemical compositions and molecular sizes[19,20]. Thus, NOM present in waters consists of both hydrophobic and hydrophilic components. The hydrophobic part is rich in aromatic carbon, having phenolic structures and conjugated double bonds, while hydrophilic NOM contains a higher proportion of aliphatic carbon and nitrogenous compounds, such as carbohydrates, proteins, sugars and amino acids. Hydrophobic acids constitute the major fraction of aquatic NOM, accounting for more than half of the dissolved organic carbon (DOC) in water[19,20]. These hydrophobic acids may be described as humic substances. The amount and characteristics of NOM in surface water depends on climate, geology and topography[4,23]. Achievement of the desired drinking water quality requires the removal of this organic matter. Thus, designing and operating a drinking water treatment plant requires emphasis on the evaluation of removal technologies for NOM. A number of investigations have dealt with these NOM removal technologies. Among the various available technologies, the most common and economically feasible method is coagulation and flocculation followed by sedimentation/flotation and filtration. Most of the NOM can be removed by the coagulation method, although, the hydrophilic, low molecular weight (LMW) fractions of NOM is apparently removed less efficiently than the hydrophobic, high molecular weight (HMW) compounds[7,14]. This preference may be due to more aromatic character, and therefore more hydrophobic nature of the latter[16]. Moreover, the hydrophobic fraction has, in general, a higher specific colloidal charge; more charged fractions are more amenable to remove[1,16]. Hence, after the coagulation process LMW and hydrophilic NOM dominate the residual organic matter[11,24]. Other treatment options for NOM removal include magnetic ion exchange resin (MIEX) technique, activated carbon filtration, membrane filtration techniques, and advanced oxidation processes[7,12,13,18,21,25,26]. The presence of NOM, as already indicated, creates serious problems to drinking water quality and its treatment processes. These problems include: (i) negative effect on water quality due to color, taste, and odor, (ii) increased coagulant and disinfectant dose requirements, which in turn results in increased sludge and potential harmful disinfection byproduct (DBP) production, (iii) promoted biological growth in distribution system, and (iv) increased levels of complexed heavy metals and adsorbed organic pollutants[7]. Especially DBP production has been of increasing concern due to their adverse health affects[17]. New compounds have been discovered as detection methods and detection levels are improving[9,15]. The most common, and among the first identified, were the trihalomethanes (THM) and haloacetic acids (HAA). More than 600 different compounds have already been identified in drinking water as a consequence of disinfection of water containing NOM[9]. All methods used for disinfection (chlorine, ozone, chlorine dioxide, chloramines, and UV-radiation) reportedly produce their own suite of DBPs and bio-reactive compounds in drinking water[15]. Present knowledge and experience show that the hydrophobic and HMW compounds of NOM are the most important pre-cursors for DBP formation[2,5]. Hydrophilic matter may also play a significant role in the formation of new compounds during disinfection, especially in waters with low humic components. Some of these are also reactive in iodine and bromine containing DBPs formation, which in turn may be even more toxic than their chlorinated counterparts[5,9,15,17].In this paper the process of gravity filtration using recycled carbon filter will be investigated with respect to the reduction of the concentration of general organic compounds, specially the ones with aromatic character, in the natural organic matter (NOM) existing in water river. No chemical or any additional treatment was used to reduce NOM. In this study, a simple lab-scale system was constructed to investigate the capacity and influence of variables such as filtration pressure, carbon granulometry and amount of carbon in the filter for the organics removal. Uberabinha River water in Uberlandia, Brazil was used. Raw water NOM concentrations are considered high enough to have the potential of yielding THM (trihalomethanes) concentrations which makes this water, without any previous treatment, inappropriate for use in treatment water station.
2. Different Methods for Characterization of NOM
2.1. Some General Parameters
- In practice, NOM[6,10] is usually represented by the measurement of total organic carbon (TOC), dissolved organic carbon (DOC), adsorption of UV-light (UV254) or chemical oxygen demand (COD). NOM is also the major contributor of the brownish yellow color in water. Measurement of color, therefore, can give some indication of the amount of NOM in water[22]. All these tests are fast and do not require sophisticated sample pretreatment or analytical equipment. Total organic carbon (TOC) is the sum of the particulate and dissolved organic carbon (DOC), existing inorganic carbon is removed by acidification. A widely accepted operational definition of DOC is the organic carbon in the water sample filtered through a 0.45 lm filter[3]. TOC and DOC are the most convenient parameters for use in the overall study of treatment processes and effects on NOM removal. Absorbance at 254 nm is typical for the aromatic groups with varying degrees of activation[8]. UV has been identified as a potential substitute measure for DOC despite tendency to only represent the aromatic character[12].
3. Adsorption onto Granular Activated Carbon
- GAC is commonly used for removing organic constituents and residual disinfectants in water supplies. This not only improves taste and minimizes health hazards, but also protects other water treatment units such as reverse osmosis membranes and ion-exchange resins from possible damage due to oxidation or organic fouling. The performance of the adsorption process is governed by a number of parameters such as carbon type, organic matter type, adsorbent particle size, water temperature and pH. Therefore, any upstream processes at a water treatment works can affect the performance of GAC. For example, coagulation preferentially removes hydrophobic NOM which in turn leaves a higher proportion of hydrophilic material in the feed which is less responsive to GAC adsorption. The specific capacity of GAC to adsorb organic compounds is related to: molecular surface attraction, the total surface area available per unit mass of carbon, and the concentration of adsorbate in the water stream. The experimental methodology used to evaluate the performance of RGAC in an adsorption column is the slow small-scale column test (SSCT). This is an inexpensive, testing method that can be used to determine the adsorptive characteristics of large scale, fixed bed adsorber using small-scale column studies. The SSCT process was developed for adsorption of organic adsorbate onto RGAC without using electricity energy as an advantage.
4. Experimental Arrangement and Procedures
- Raw water used for lab-scale experiments was from Uberabinha River located at Uberlandia – Brazil. Water samples for this study were collected in the morning every day and used immediately in the experiments. The process used was gravitational filtration with filters of recycled activated carbon. The volume of influent water required is small enough so that an adequate volume of water can be transported to a laboratory.The lab-scale installations are schematically shown in Fig. 1. From an 11-L storage reservoir or the feed tank, the raw water was, firstly, passed through a valve. This valve only controls the beginning and the end of the process. Secondly, the water was conducted across the filter in downstream direction, that was
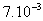 m in diameter and varying
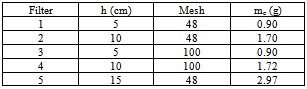
m in diameter and varying 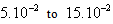 m in height (h) which results in a carbon mass (mc, in grams) between 0.9 and 3.0 g.. Recycled carbon used had size of 143 and 298 μm or mesh 100 and 48. Table 1 presents the characteristics of the filters used. The system was operated under conditions at room temperature of 21.0 ± 0.5 0C measured using a thermometer inserted in the tank. The percolation velocity was in the range of 0.7-5.5 m h-1 taking into account the pressure drop and the saturation state of the filter. Three different operating pressures were used for each filter: 58, 87 and 126 cm H2O. The one was kept constant during the filtration process. Samples of permeate or filtered were collected and weighted (Bioprecisa (±10-4g)) with the time duration of approximately 1/1 h for a period of around 7-11 hours.
m in height (h) which results in a carbon mass (mc, in grams) between 0.9 and 3.0 g.. Recycled carbon used had size of 143 and 298 μm or mesh 100 and 48. Table 1 presents the characteristics of the filters used. The system was operated under conditions at room temperature of 21.0 ± 0.5 0C measured using a thermometer inserted in the tank. The percolation velocity was in the range of 0.7-5.5 m h-1 taking into account the pressure drop and the saturation state of the filter. Three different operating pressures were used for each filter: 58, 87 and 126 cm H2O. The one was kept constant during the filtration process. Samples of permeate or filtered were collected and weighted (Bioprecisa (±10-4g)) with the time duration of approximately 1/1 h for a period of around 7-11 hours.
|
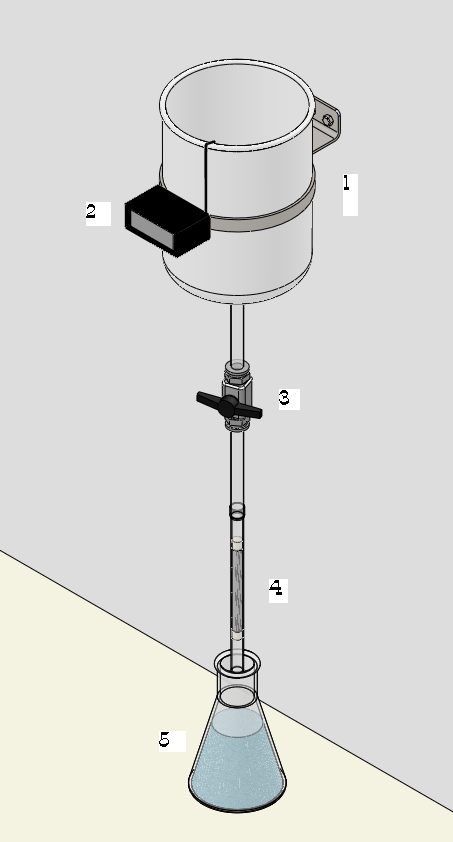 | Figure 1. Equipment used for the gravity filtration. (1) Feed tank, (2) Thermometer, (3) Valve, (4) Filter, (5) Collector |
5. Results and Discussion
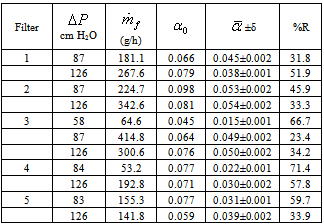
- Three parameters were investigated for their effect on the performance of RGAC columns, namely RGAC particle size, mass (or height) of carbon and pressure drop. The range of particle size investigated had a geometrical mean size of 143 and 298 μm. Five filters and eleven experiments were conducted to evaluate the effect of parameters cited below on the breakthrough behavior. In Table 2, observing results for mesh 48 or 100 and = 87 cm H2O, we can conclude that as height (h) increased, organic compounds removing has risen. Also, despite filter 4 at pressure drop of 84 cm H2O was the best for decreasing NOM concentration, it had the lowest production of filtered. In addition, among all filters and operating conditions experimented, if you have to decide for one filter which conciliates filtered production and better organic compounds removing, it is recommended filter 1 (h=5 cm, 126 cm H2O) or filter 2 (h=10 cm; 84 cm H2O). However, when high rates of filtered is the major objective, filter 3 (h=5 cm, 87 cm H2O) is the most appropriate; otherwise if organic compounds removing is the goal, filter 4 is the suitable one.The average percentage of general organic substances removed (%R) was calculated for each experiment based on the medium value of filtered absorbance according to the following equation:
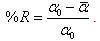
|
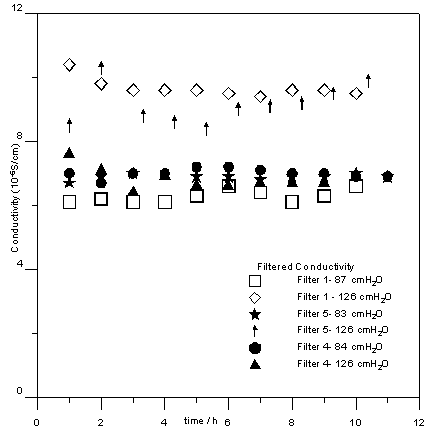 | Figure 2. Filtered conductivity versus time for different filters and pressure drop |
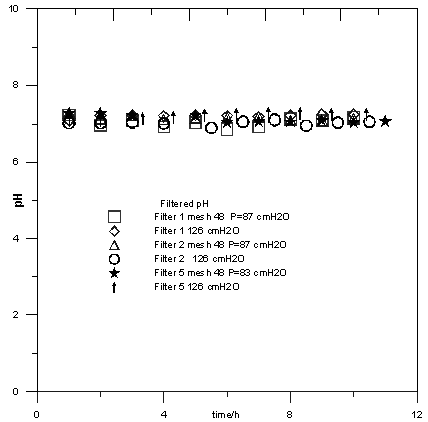 | Figure 3. Influence of pressure and carbon mass in the filter under filtered pH |
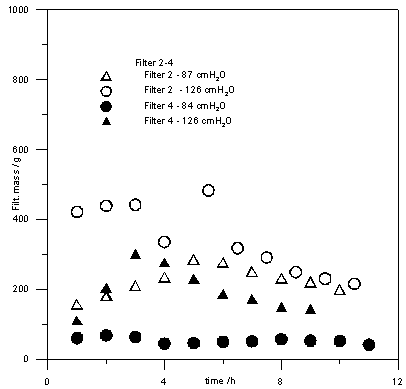 | Figure 4. Filtered mass as a function of time for filters 2 and 4 |
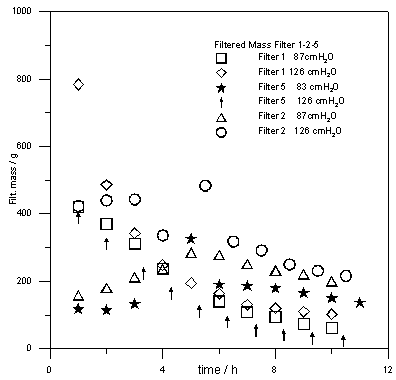 | Figure 5. Filtered mass as a function of time for different filters |
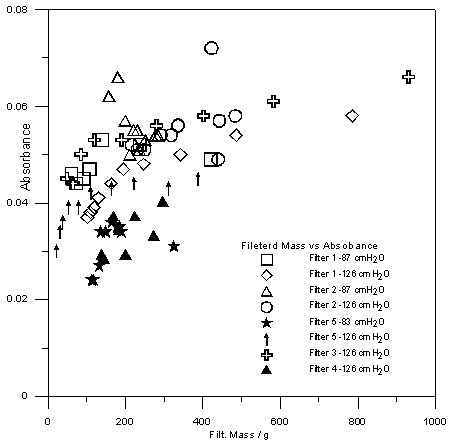 | Figure 6. Influence of granulometry and mass of carbon and pressure in the amount of filtered mass collected |
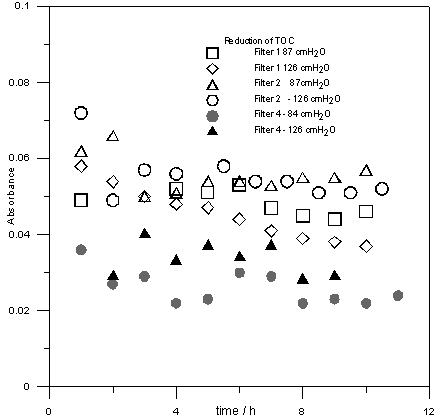 | Figure 7. Influence of granulometry and mass of carbon and pressure in the reduction of MON comparing the filters 1,2 and 4 |
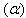 varies all filtration processing. The variation is less pronounced for higher amount of filtered. In Fig. 7, it can be seen that dissolved organic compounds reduction remains practically constant after eight hour filtering. Also, when the carbon granulometry is reduced from mesh 48 to 100, it is noted that the removing capacity is decreased in the half. The amount of activated carbon in the filter seemed not influencing substantially in the process.
varies all filtration processing. The variation is less pronounced for higher amount of filtered. In Fig. 7, it can be seen that dissolved organic compounds reduction remains practically constant after eight hour filtering. Also, when the carbon granulometry is reduced from mesh 48 to 100, it is noted that the removing capacity is decreased in the half. The amount of activated carbon in the filter seemed not influencing substantially in the process.6. Conclusions
- In general, for small amounts of river water, general organic compounds concentrations were reduced using gravity filtration with recycled carbon filter.Dissolved organic compounds adsorption capacity onto RGAC is higher for smaller GAC particle size (Filter 3 and 4) and smaller pressure drop. The most suitable RGAC bed parameters were found to be a mean particle size of 143 μm. It could be attributed for its higher surface available.Considering that no changes were observed in the conductivity and pH of the filtered, we concluded that this process can not be recommended as an option when ionic substances have to be removed from the raw water.Raw water treatment by RGAC adsorption without pre-treatment is currently a future option related to the raw water trophic state. The choice of a particular system would be based on ad hoc cost-effectiveness study at the time.
ACKNOWLEDGMENTS
- The authors would like to thank PROPAP-UFU for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML