-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2013; 3(4): 179-186
doi:10.5923/j.ajee.20130304.03
Adsorption of Chromium (VI) from Aqueous Solutions by Lebanese Prunusavium Stems
Nour Al-Afy1, Akram Hijazi1, Hassan Rammal1, Mohamad Reda2, Houssein Annan1, Joumana Toufaily2, 3
1Doctoral School of Science and Technology, Research Platform for Environmental Science (PRASE), Lebanese University, Lebanon
2Laboratory of Materials, Catalysis, Environment and Analytical Methods (MCEMA), Lebanese University, Lebanon
3School of Mechanical and Materials Engineering, Washington State University, Washington State, United State of America
Correspondence to: Hassan Rammal, Doctoral School of Science and Technology, Research Platform for Environmental Science (PRASE), Lebanese University, Lebanon.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A new biosorbent fromthe stems of Lebanese Prunusavium(sweet cherry) was used to remove chromium (VI) from aqueous solutions. The biosorption of chromium (VI) was found to be dependent on solution pH, biosorbent dose, initial metal ion concentrations, temperatureand contact time. Maximum adsorption capacity (78.43 %) of chromium was obtained at pH 2, contact time 1 hour at 25℃. The experimental equilibrium biosorption data were analyzed by two widely used two-parameters, Langmuir and Freundlich isotherm models. The Langmuir model gave a better fit than Freundlich model. It was concluded that stems ofPrunusaviumwere cost effective, environmental friendly and have a great potential for use as adsorbent in removing Cr (VI) from aqueous solution.
Keywords: Prunusavium, Chromium (VI), Adsorption, Langmuir and Freundlich Isotherm Models
Cite this paper: Nour Al-Afy, Akram Hijazi, Hassan Rammal, Mohamad Reda, Houssein Annan, Joumana Toufaily, Adsorption of Chromium (VI) from Aqueous Solutions by Lebanese Prunusavium Stems, American Journal of Environmental Engineering, Vol. 3 No. 4, 2013, pp. 179-186. doi: 10.5923/j.ajee.20130304.03.
Article Outline
1. Introduction
- Heavy metals are toxic for the environment that occurs in much industrial waste water.Therefore, their removal from waste water is essential to protect human health and environment[1]. Chromium is highly toxic and occurs in the environment in two valence states: Cr (III) and Cr (VI). They are getting from natural or industrial sources.Cr (III) is less toxic than Cr (VI). Hence hexavalent chromium Cr (VI) moves rapidly through waste water and soil also it is strong oxidizing agent that can be absorbed by the skin[2]. Acute exposure to Cr (VI) causes dermatitis, diarrhea, nausea, kidney and liver damage, respiratory problems and internal haemorrhage[3] while inhaling Cr (VI) may lead to acute toxicity, nasal septum ulceration and perforation,respiratory irritation and asthma. However the kidney and liver dysfunction may be occur due to Cr (VI) injection. Skin contact may cause poisonous damage for system, interference with the healing of cuts or scrapes or even a severe burn[4,5,6]. If chromium is not treated it may cause severe chronic allergic contact, dermatitis and ulceration. Permanent damage may be caused by eye exposure to Cr (VI)[7]. Different physiochemical technique are being used to reduce the toxicity of chromium from waste water such as reverse osmosis, ion exchange, chemical precipitation, membrane filtration, solvent extraction, electrolytic methods and activated carbon adsorption[8,9,10] but they have incomplete removal and high material cost. Several researches are done in natural adsorbent to remove chromium from aqueous solutions such as plant materials likecrassipes[11], Casurinaequisetifolia leaves[12], Panki Thermal Power Station (PTPS) fly ash[13], water lilies (Nymphaeaspontanea)[14], Helianthus annuus (sun flower stem)[15], aquatic weeds[16], Loliumperenne leaves[17], Ocimumamericanum[18], Posidonia oceanic fibers[19], etc…In the present work, the stems of the Lebanese Prunusaviumhas been used as adsorbent to remove Cr (VI) from waste water by varying the experimental conditions such as contact time, initial metal ion concentration, temperature and pH, parameters could be applied in the reduction of chromium pollution in the environment.
2. Materials and Methods
- The raw P.avium (sweet cherry) stems were collected from a local Lebanese plantation. The stems were thoroughly rinsed with water. Then, they were dried at room temperature for 10 days. After that, the dried stems were grounded to a fine powder in a grinding mill to get size 0.25 μm.
2.1. Chemicals
- The stock solution was prepared by dissolving 0.15 g of K2Cr2O7 in 1l deionized water. All the required working solutions were prepared by diluting the stock solution with deionized water.Samples for Fourier transform infrared (FT-IR) were prepared by diluting the adsorbent to 5% in KBr and cast in disks for analysis. Analysis of standards and simulated samples was done using an AA-140 atomic absorption spectrometer (AAS).
2.2. Batch Studies
2.2.1. Batch Experiments for Cr (VI)
- Batch experiments were carried out using a series of erlenmeyer flask of 50 ml capacity. Batch experiments were prepared to study the effect of initial Cr (VI) concentration, pH, contact time, adsorbent dose and temperature on adsorption of the Cr (VI) ions from its solution. All the adsorption experiments were carried out at room temperature except where the effect of temperature was being investigated. The initial pH was adjusted with HNO3(1 M) or NaOH(1 M) solutions.
2.2.2. The Effect of Metal Ion Concentration on Adsorption
- 0.5 g of the adsorbent was shacked with 50 ml of varying concentrations (25, 150, 300, 400 and 600 mg/l) of Cr(VI) solution. The mixture was continuously agitated at 25±2℃ with a shaker at 700 rpm. The pH of the solution was adjusted to a pH 2. After the established contact time (1 hour) was reached, the suspension was filtered in 2 steps: first by Buchnerfiltration then by 0.45 μm filter. After that, the final concentration of Cr(VI) in the filtrate was determined using AAS. The adsorbed amount was determined from the difference between the initial and residual concentrations of Cr (VI) in the liquid phase.
2.2.3. The Effect of pH on Adsorption of Cr (VI) Ions
- The effect of pH on adsorption was investigated in the pH range 2-12 at 25 ±2℃. The initial pH of the Cr (VI) solution was adjusted from pH 4 by adding few drops of HNO3(1 M) to get a pH 2. Solutions of pH between 6 and 10 were prepared by adding few drops of NaOH (1 M)to a pH 4.
2.2.4. The Effect of Contact Time on the Cr (VI) Adsorption
- Theeffect of the contact time was also performed with 50 ml of 150 mg/l Cr (VI) solution at a pH2. 0.5g of adsorbent at 25±2℃ was added to aqueous solution in respect to the following times: 30, 60, 120, 180, 240 min and mixed with a shaker set at 700 rpm. After that, the samples were filtered and the filtrate was then analyzed by AAS in order to determine the adsorption capacity.
2.2.5. The Effect of Temperature on the Metal Adsorption
- Experiments for the effect of temperature were also performed following the same procedure by fixing all other parameter and varying only the temperature ranges: 0, 25, 35, 45 and 55℃.
2.2.6. The Effect of the Adsorbent Dose on the Removal of Cr(VI) Ions
- Adsorption dose experiments were also performed following the same procedure as described earlier at pH 2, 150 mg/l Cr (VI), 25 ± 2℃, 700 rpm, with the following adsorbent masses: 0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.4, 1.6, 1.8, 2, 2.2 and 2.4 g after 1 h was reached, the samples were filtered and the filtrate analyzed by AAS.
3. Results and Discussion
- When P.avium stem powder was tested for its abilityto absorb Cr (VI) ion from aqueous solution,initial pH 2 was used for most experiments. Theeffects of the following experimental parameters (pH, initial concentration, contact time, temperature and the adsorbent dosage)onadsorption were studied. Thepercentage of the uptake or adsorption of Cr (VI) wascalculated using the following equation:
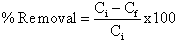 | (1) |
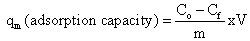 | (2) |
3.1. FT-IR and XRF Analysis of Adsorbent
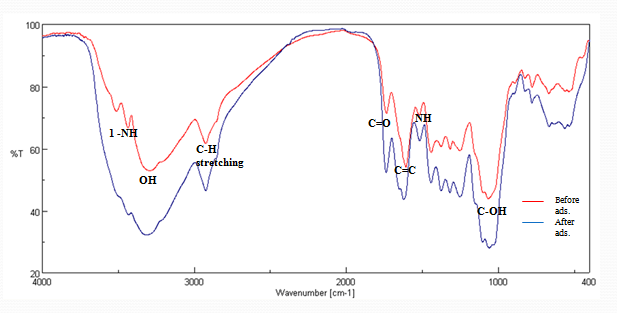
- The FT-IR spectrum (Figure 1) of P.avium was used toinvestigate the functional groups present on the P.avium that could be responsible for the removal of heavy metalspecies[20]. The spectrum of the adsorbent was measured within the range of 4000-400 cm-1 wave number. The comparison of the FT-IR spectra has been done before and after loading with Cr (VI). P.avium stems show a number of absorption peak that reflect its complex nature. Two peaks at 3513 cm-1 and 3436 cm-1 are due to the presence of N-H bond stretching (primary amine). A broad peak at 3292 cm-1 is due to the existence of OH group. The absorption peak at 2924 cm-1 could be assigned to C-H stretching vibration, 1736 cm-1 to ester carbonyl, 1608 cm-1 to C=C, 1520 cm-1 to N-H,1066 cm-1 to C-O.After adsorption, a broad peak at 3466 cm-1 corresponds to the overlapping of OH and NH peak. This phenomenon may be attributed to the water molecule directly interacting with amide.
 | Figure 1. FT-IR spectrum of stems of Prunusavium before use and after adsorption |
 | Figure 2. XRF spectrum of stems of Prunusaviumpowder before use |
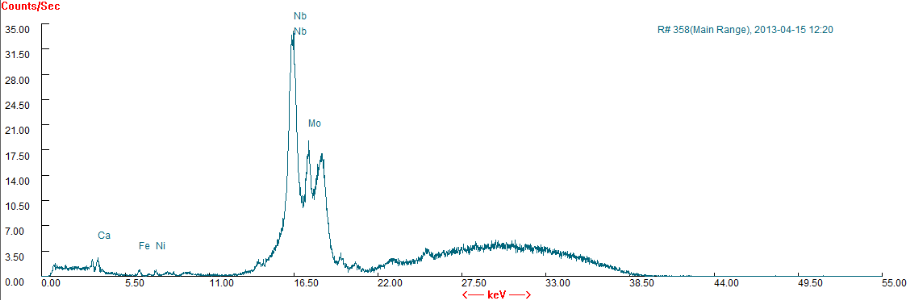
- After Cr (VI) binding, a change of peak position occurs (3437-3435cm-1, 3292-3290 cm-1, 1736-1738 cm-1, 1253-1252 cm-1, 1066-1056 cm-1, 824-811 cm-1, 536-558 cm-1).The shift in the wave number corresponds to the change in the energy of the functional groups that indicates the existence of Crbinding process done on the surface of P.avium stem powder[21].The XRF spectrum has been done to show the metals composition in the stems of P.avium. It was consisting of Ca, Fe, Ni, Nb, Mo among others as shown in figure 2.
3.2. Adsorption of Cr (VI)
3.2.1. Effects of Cr (VI) Concentration
- The effect of varying concentration of Cron the adsorption capacity while fixing the other four parameters is shown in figure 3. The adsorption of Cr by P.aviuminitially increases rapidly with the concentration of Cr(VI) ion until it reaches a maximum of 13.46 mg/g at 400 mg/l (figure 3). The initial rapid increase in the removal of Cr (VI) can be attributed to the interactions between the metal ions and the active sites of the P.avium. At higher concentrations, the adsorption capacity remains constant due to saturation of the binding sites of the P.avium so the Cr left unadsorbed[2]. From similar studies conducted by Malkoc et al.[22], the adsorption of Cr (VI) on pomace at 25℃ gave qm values of 6.10, 10.34, 10.80 and 2.12 mg/l for solutions with concentrations of 50, 100, 150 and 200 mg/l, respectively. These results are similar to the observations made in our study where the adsorption capacity of tassel for Cr (VI) was in the range 1.8 – 10 mg/g. The work done by Dubey and Krishna[23] found that the maximum adsorption capacity of most investigated biosorbents was in the range of 1.6 mg/g – 13.4 mg/g for unmodified biosorbents.
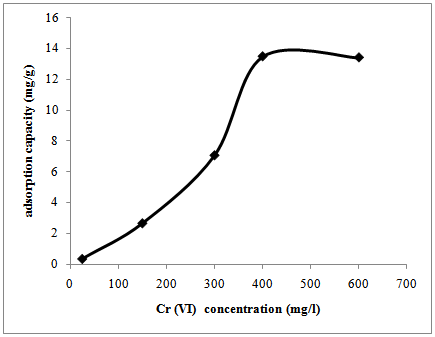 | Figure 3. Effect of initial concentration on the adsorption capacity of Prunusavium |
3.2.2. Effects of pH on Cr (VI) Ion Adsorption
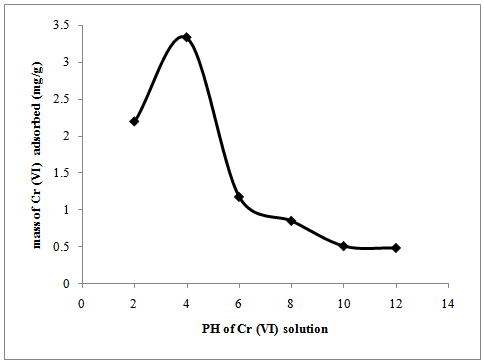
- The effect of the pH on the adsorption of Cr (VI) is presented in figure 4. The pH of the aqueous solution is an important parameter which controls the adsorption process, since it affects the solubility of the metal ions concentration of the counter ions on the functional groups of the adsorbent and the degree of ionization of adsorbate during reaction[24]. The pH was varied from 2 to 12, while the other four parameters (adsorbent dosage, initial ion concentration, contact time and temperature) were fixed (0.5 g, 150 mg/l, 1 h and 25±2℃ respectively). Generally, the adsorption capacity of P.avium on Cr (VI) increases between pH2 and pH 4 to reach optimum at pH 4.From Figure 4 it can be seen that there is a sharp decrease of adsorption of Cr (VI) from pH 4 reaching the minimum at pH 12. The sorption capacity of Cr (VI) at pH 4 by P.avium was 3.3 mg/g, which reduced to 0.49 mg/g at pH 12. Where at low pH, there is a large number of H+, which in turn attracts the negative charges of the adsorbent surface thereby reducing hindrance to the diffusion of dichromate ions[25]. Hence, the sorption increases with the increase in the acidity of the solution. As the pH increases, the amount of OH- ions increases and overall charge on the adsorbent surface becomes negative which causes hindrance in the sorption of the negative charges of Cr (VI) ions like Cr2O72-, CrO42- and HCrO4– leading to a decrease in the sorption of Cr (VI) at high pH[25]. The other possible mechanism of adsorption of Cr (VI) is through the oxidation of the adsorbent surface to groups like carbonyl (C=O), carboxylic (COOH) and hydroxyl (OH) groups. Then the Cr (III) reduced from Cr (VI) which is positively charged and will be attracted to negatively charged functional groups generated on the surface of adsorbent by oxidation[2].
3.2.3. Effect of Contact Time on Removal of Cr (VI) Ion
- The effect of contact time on the adsorption of Cr (VI) from its solution is presented in figure 5. There is an increase in the % removal of Cr (VI) to reach optimum (55.8 %) after 1 hour.This could be possibly the time required for the equilibrium to be established. After that, a general decrease in the % adsorption of Cr (VI) with time has been noted.
3.2.4. Effect of Temperature on Cr (VI) Adsorption
- The adsorption of Cr (VI) from aqueous solutions at different temperatures was investigated. Samples were subjected to temperatures ranged from 0 to 55℃ as shown in figure6. As the temperature increases the adsorption capacity increases until it reaches maximum adsorption efficiency of 2.5 mg/g (53.83 %) at 35℃ and thereafter it decreased gradually. From the effect of temperature, studies conducted byMalkoc et al.[22] on the adsorption of Cr (VI) observed that the adsorption capacity of Cr (VI) has increased with temperature. This was observed in the temperature range 45℃ – 60℃ for the concentration 50 to 200 mg/l. When the solution temperature was increased the oxidizing power of the Cr (VI) ionswas increased. The ions were then reduced to Cr (III) and were bound strongly on the organic material. However, overheating may cause desorption kinetics dominating[2].
3.2.5. Effect of Adsorbent dose on Adsorption of Cr (VI) Ion
- The adsorbent dosage isanother important parameter which influences the extent of metal uptake from the solution and thus the effect is shown in figure 7. The % removal of Cr by P.avium at different adsorbent dose (0.2 to 2.4 g in 50 ml) for Cr (VI) concentration (150 mg/L) and fix other parameter: temperature 25 ± 2℃, contact time 1 h, agitation speed 700 rpm and pH 2. The result illustrated in figure 7 shows that as the adsorbent dose increases the % removal increases to reach 74% of Cr (VI) adsorbed at 0.8 g and then remains constant. This result supposes the presence of a relationship between the adsorbent dosage and the removal efficiency due to an increase in the number of adsorption sites, however after equilibrium was reached the increasing in the adsorbent dosage will has no effect[1].
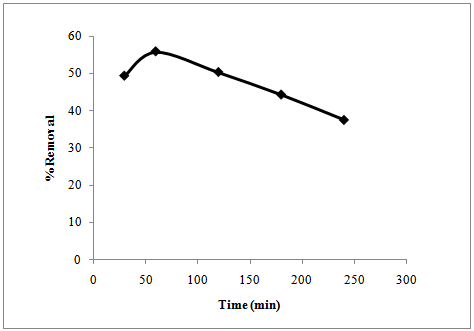 | Figure 5. Effect of contact time on the adsorption of Cr (VI) |
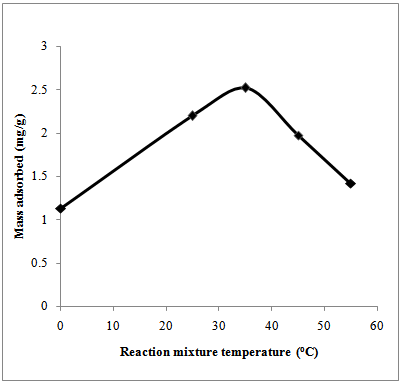 | Figure 6. Effect of temperature on Cr (VI) adsorption |
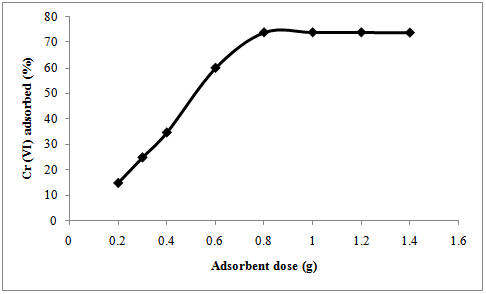 | Figure 7. Effect of adsorbent dose on adsorption of Cr (VI) |
3.3. Biosorption Isotherms
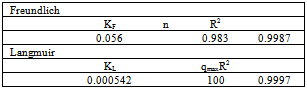
- Adsorption isotherms are essential for understanding the mechanism of an adsorption system. Since they represent the amount of compounds adsorbed on a surface as a function of concentration at a constant temperature[24]. Two isotherms models were tested:1. Freundlich isotherm model is the well-known earliest relationship whichdescribes the adsorption process. It can be applied to non-ideal sorption on heterogeneous surfaces as well as multilayer sorption[26].This isotherm is expressed by the following linear equation:
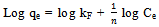 | (3) |
|
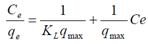 | (4) |
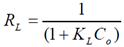 | (5) |
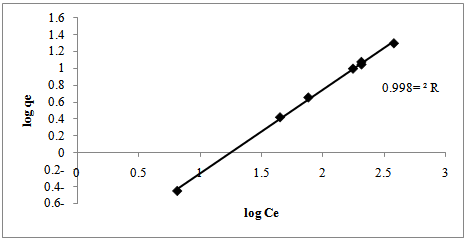 | Figure 8. Freundlich isotherm for Cr (VI) biosorption into Prunusavium stems |
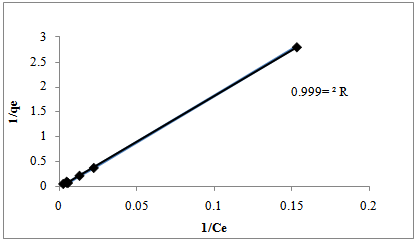 | Figure 9. Langmuir isotherm for Cr (VI) biosorption onto Prunusavium stems |
4. Conclusions
- This study investigated the adsorption of Cr (VI) ions from aqueous solutions onto dried Prunusavium stems that is dependent on biosorption process such as initial metal ions concentration, pH, adsorbent dose, temperature and contact time. To provide best correlation for biosorption of Cr (VI) ions onto P.avium stems, Freundlich and Langmuir biosorption isotherm were demonstrated. From this study, it was observed that stems of P.avium can be used as an alternative low cost, eco-friendly and effective adsorbent for treatment of waste water containing Cr (VI) ions. Other heavy metals adsorbed by stems of P.avium are under study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML