-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2012; 2(6): 188-195
doi: 10.5923/j.ajee.20120206.07
Biosorption Studies of Cr(VI) Ions from Electroplating Wastewater by Walnut Shell Powder
Ahlam M. Farhan 1, Nida M. Salem 2, Ammar H. Al-Dujaili 3, Akl M. Awwad 4
1Department of Chemistry, College of Science for women, University of Baghdad, Baghdad, Iraq
2Plant Protection Department, Faculty of Agriculture, Jordan University, Amman, Jordan
3Department of Chemistry, College of Education, Ibn Al-Haytham, University of Baghdad, Baghdad, Iraq
4Royal Scientific Society, Amman, Jordan
Correspondence to: Akl M. Awwad , Royal Scientific Society, Amman, Jordan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Biosorption of Cr(VI) ions from aqueous solutions and electroplating wastewater by walnut shell powder has been investigated in a batch biosorption process. The biosorption of Cr(VI) ions was found to be dependent pH, initial chromium ion concentrations, biosorbent dose, contact time and temperature. The experimental equilibrium biosorption data were analyzed by Langmuir, Freundlich, and Temkin isotherm models. The Langmuir model gave a better fit than the Freundlich and Temkin models. The maximum biosorption capacity calculated from the Langmuir isotherm was 138.89 mg/g at optimum conditions. The kinetic studies indicated that the biosorption process of the chromium ions followed well pseudo-second-order model. The negative values of ∆Go (-3.51 kJ/mol) and positive value of ∆Ho (12.95 kJ/mol) revealed that the biosorption process was spontaneous and endothermic. Biosorption process was successfully applied to the treatment of an electroplating wastewater sample, where the concentration of chromium (VI) ions, organic materials and COD were effectively reduced. According to the sorption capacity, walnut shell powder considered as an effective, low cost, and environmentally friendly biosorbent for the removal of Cr(VI) ions from aqueous solution and electroplating wastewater.
Keywords: Biosorption, Electroplating Wastewater, Walnut Shell Powder, Cr(VI) Ions
Cite this paper: Ahlam M. Farhan , Nida M. Salem , Ammar H. Al-Dujaili , Akl M. Awwad , "Biosorption Studies of Cr(VI) Ions from Electroplating Wastewater by Walnut Shell Powder", American Journal of Environmental Engineering, Vol. 2 No. 6, 2012, pp. 188-195. doi: 10.5923/j.ajee.20120206.07.
Article Outline
1. Introduction
- The major sources of Cr(VI) release into the environment by waste streams are electroplating, leather tanning, paint dyes and textiles industries. Chromium in the aquatic environment has been classified in group A of human carcinogens by United States Environmental Protection Agency (USEPA). Therefore, USEPA has limited the industrial charge of Cr(VI) to surface water up to < 0.05 mg/L. The most commonly methods used for the removal of Cr(VI) from aqueous solutions and industrial effluents are chemical reduction, electrochemical precipitation, chemical precipitation, chemical oxidation-reduction, ion exchange, reverse osmosis, electro dialysis, evaporation and adsorption. These methods have significant disadvantages, including high energy requirements, incomplete metal removal, generation toxic sludge needs treatment and expensive equipments. Biosorption of toxic heavy metals by biomaterials has been suggested as a potential alternative to the conventional methods for recovery of toxic heavy metals from wastewater[1-2]. Many biomaterials such as micro-algae[3], fungi[4], fungal biomass[5-7], walnut hull [8], almond green hull[9], Helianthus annuus stem waste [10], banana skin, green tea waste, oak leaf, walnut shell, peanut shell and rice husk[11], Ocimum americanum L. seed pods[12], chemically modified coir pith[13], groundnut shell[14], activated carbon from tamarind wood activated with zinc chloride[15], olive stone[16], grape waste[17], hazelnut[18], walnut, hazelnut and almond shell [19], pistachio hull waste[20], agriculture wastes, carbons [21], rice husk-based active carbon[22], fruit shell of gulmohar[23], coconut husk[24], husk of bengal gram[25], eucalyptus bark[26], agricultural waste biomass[27], pine needles[28], sugar cane bagasse[29], leaf mould[30] and waste pomace of olive factory[31].
2. Materials and Methods
2.1. Adsorbent
- Walnut fruit was collected from a local fruit field in the north part of Iraq and the brown hard shell of the walnut fruit was thoroughly rinsed with distilled water to remove dust and soluble materials. Then it was allowed to dry at room temperature. The dried walnut shell was crushed and grounded to a fine powder in a grinding mill (Retsch RM 100) and sieved to get size fraction < 44 µm, and then dried in an oven at 60℃ for 24 h. The dried biosorbent was stored in a desiccator and used for the batch experiments.
2.2. Materials
- Analytical grade of K2Cr2O7, HCl and NaOH were purchased from Fluka AG. A stock solutions of 1000 mg/L of Cr(VI)) ions were prepared by dissolving KCr2O7 in 1000 mL of distilled deionized water. Desired test solutions of Cr(VI) ions were prepared using appropriate subsequent dilutions of the stock solution. The range of concentrations of Cr(VI) ions prepared from standard solution varies between 10 and 100 mg/L. Before mixing the adsorbent, the pH of each test solution was adjusted to the required value with 0.1 M NaOH or 0.1 M HCl.
2.3. Electroplating Wastewater
- The chromium rinse water samples were collected from an electroplating factory, Jordan. The electroplating wastewater collected was analyzed for different physico-chemical properties, Table 1, viz. total dissolved solids, hydrogen ion concentration, COD, conductivity, sulphate, chlorides, phosphate, chromium and zinc (II). The concentration of each of the component was determined by standard analysis methods for water and wastewater.
2.4. Analysis
- The concentrations of Cr(VI) ions in the solutions before and after equilibrium were determined by AAS6300 atomic absorption spectrometer (Shimadzu). The pH of the solution was measured with a WTW pH meter using a combined glass electrode. Fourier transform infrared spectroscopy, The chemical oxygen demand (COD) of electroplating wastewater was analyzed by a COD reactor (Thermoreakter TR 420, Merck), Conductivity was measured by a WTW conductometer (inoLab Cond. Level 1). FT-IR (IR Prestige-21, Shimadzu) was used to identify the different chemical functional groups present in the walnut shell powder and also used to determine the functional groups which are responsible for the chromium binding with walnut shell powder. The analysis was carried out using KBr and the spectral range varying from 4000 to 400 cm−1.
|
2.5. Batch Biosorption Experiments
- Batch biosorption experiments were conducted by mixing biosorbent with Cr(VI) ion solutions with desired concentration in 250 mL glass flask. The glass flasks were stoppered during the equilibration period and placed on a temperature controlled shaker at a speed 120 r/min. The effect of pH on the equilibrium biosorption of Cr(VI) was investigated by mixing, The amount of biosorption was calculated based on the difference between the initial (Co, mg/L) and final concentration (Ce, mg/L) in every flask, as follows:
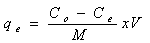 | (1) |
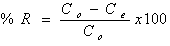 | (2) |
3. Results and Discussion
3.1. FTIR Spectral Analysis
- The FT-IR spectra of walnut shell powder (before and after sorption of chromium) were used to determine the vibrational frequency changes in the functional groups in the adsorbent. The FT-IR spectra of the adsorbent display a number of absorption bands indicating the complex nature of studied adsorbent. In native walnut shell powder, the broad absorption band at 3430 cm-1 is indicative of the existence of bonded hydroxyl group. The absorption peak around 2905, 1744 and 1053 cm-1 is assigned to –CH, C=O and C-O stretching, respectively. The peak observed at 1623 cm-1 corresponds to C=C stretching that may be attributed to the lignin aromatic group (Fig. 1). The additional peak at 604 cm-1 can be assigned to bending modes of aromatic compounds. The –OH absorption band was observed to shift to 3427 cm-1 when walnut shell powder is loaded with Cr(IV). The –CH stretching band also shifted to 2928 cm-1. It seems that these functional groups participate in metal binding. The changes in FT-IR spectra confirm the complexation of Cr(VI) with functional group present in the adsorbent.
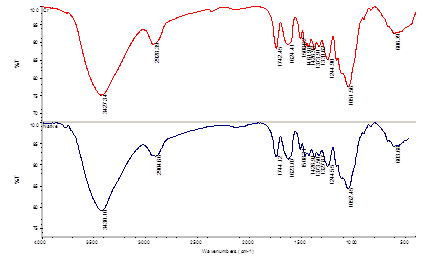 | Figure 1. FT-IR spectra of native walnut shell and loaded-Cr(VI) sample |
3.2. Effect of pH
- The effect of pH on the biosorption of Cr(VI) ions onto walnut shell powder was studied at pH 1.0–6.0. The maximum biosorption was observed at pH 2.0 for Cr(VI) ions. Therefore, the remaining all biosorption experiments were carried out at this pH values. pH is a vital parameter affecting the biosorption process. It was observed that the biosorption efficiency of Cr(VI) decreased as the pH increased. The maximum uptake capacity of the walnut shell powder was 138.89 mg/g for an initial Cr(VI) concentration of 40 mg/L at pH of 2.0 and 30℃. The increased binding of Cr(VI) at low pH can be explained by two factors. First, biosorption of Cr(VI) at pH 2.0 suggests that the negatively charged chromium species (chromate/dichromate in the medium) bind through electrostatic attraction to positively charged functional groups on the surface of biosorbent. As the pH increased, the overall surface charge on the cells became negative and biosorption decreased. In alkali conditions, carboxylate group exists in deprotonated form and has net negative charge. As a result, the surface charge of the biosorbents become negative and biosorption of Cr(VI) decreases. Secondly, the solution chemistry of Cr(VI) ions can affect the biosorption process. Previous studies[9, 18] showed that chromium exhibits different types of pH dependent equilibria in solutions. Sorbate and chromium form stable complexes such as Cr2O72-, HCrO4-, CrO4- and HCr2O7-, the fraction of any particular species is dependent on chromium concentration and pH. In low chromium concentration, the main fraction is HCrO4- with pH below 5.0, whereas the CrO4- increases with increase of pH value and becomes the main form with pH above 6.0.
3.3. Effect of Initial Metal Concentration
- Chromium (VI) ions biosorption was studied in batch experiments (pH 2.0) using different initial Cr(VI) concentrations of 10, 20, 40, 60, 100 mg/L. The equilibrium uptake of the biosorbent was 11.44, 12.98, 16.26, 17.42 and 18.65 mg/g at initial concentration 10, 20, 40, 80 and 100 mg/L chromium ions, respectively. It is evident that the amount of chromium adsorbed onto the biosorbent increases gradually with an increasing concentrations of Cr(VI). The increase of adsorption yield with the increase in chromium ion concentration is probably due to higher interaction between the metal ions and metal sequestering sites of biosorbent.
3.4. Effect of Contact Time
- The rate of biosorption is important for designing batch biosorption experiments. Therefore, the effect of contact time on the biosorption of Cr(VI) was investigated. The biosorption of Cr(VI) increased considerably until the contact time reached 60 min at 30oC. Further increase in contact time did not enhance the biosorption, so, the optimum contact time was selected as 60 min for further experiments.
3.5. Effect of Adsorbent Dose on Biosorption
- The biosorbent dosage is an important parameter because this determines the capacity of a biosorbent for a given initial concentration. The biosorption efficiency for Cr(VI) ions as a function of biosorbent dosage was investigated. The percentage of the Cr(VI) biosorption steeply increases with the biosorbent loading up to 0.5 g/0.1 L. This result can be explained by the fact that the biosorption sites remain unsaturated during the biosorption reaction whereas the number of sites available for biosorption site increases by increasing the biosorbent dose. The maximum biosorption 94.82% for Cr(VI) was attained at biosorbent dosage, 0.5 g/0.1 L. Therefore, the optimum biosorbent dosage was taken as 0.5 g/0.1 L for further experiments.
3.6. Effect of Temperature
- The effect of temperature was studied using initial Cr(IV) concentration 40 mg/L at pH 2.0. The results showed that, the rate of Cr(IV) biosorption increased with an increased in temperature from 20℃ to 40℃. At the temperature range (25 – 35℃) the absorption capacity is nearly constant, then further increase in temperature result in slight decrease in Cr(IV) biosorption.
3.7. Biosorption Isotherms
- An adsorption isotherm describes the fraction of sorbate molecules that are partitioned between liquid and solid phases at equilibrium. Adsorption of Cr(VI) ions onto walnut shell powder was modelled using three adsorption isotherms:
3.7.1. Langmuir Isotherm
- The Langmuir isotherm assumes monolayer adsorption on a uniform surface with a finite number of adsorption sites[32]. Once a site is filled, no further sorption can take place at that site. As such the surface will eventually reach a saturation point where the maximum adsorption of the surface will be achieved. The linear form of the Langmuir isotherm model is described as:
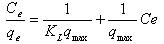 | (3) |
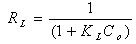 | (4) |
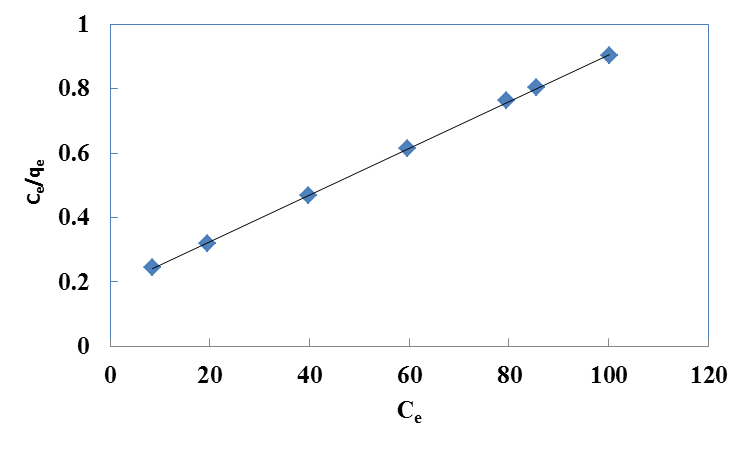 | Figure 2. Langmuir isotherm for Cr(VI) ions onto walnut shell powder |
3.7.2. Freundlich Isotherm
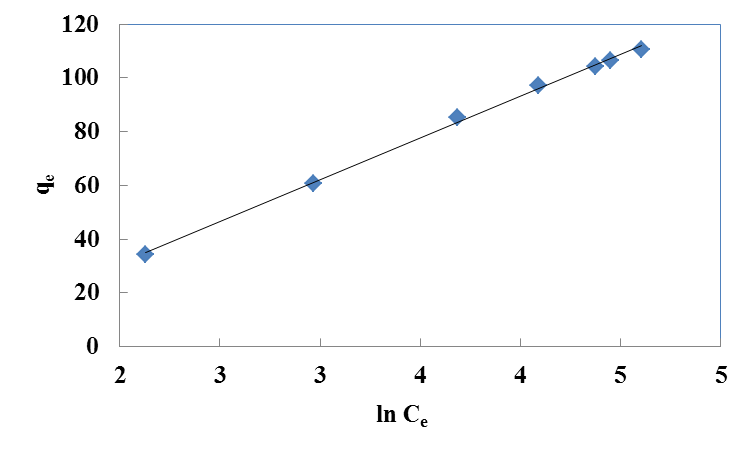
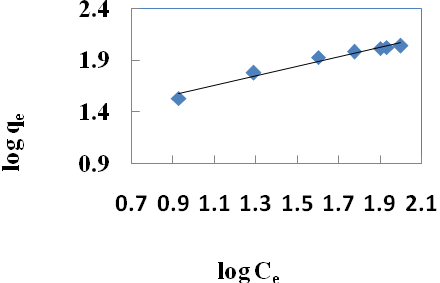 | Figure 3. Freundlich isotherm for Cr(VI) ions onto walnut shell powder |
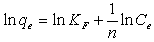 | (5) |
 | Figure 4. Temkin isotherm for Cr(VI) ions onto walnut shell powder |
3.7.3. Temkin Isotherm
- Temkin isotherm equation[34] assumes that the heat of biosorption of all the molecules in the layer decreases linearly with coverage due to adsorbent-adsorbate interactions and that the adsorption is characterized by a uniform distribution of the binding energies up to some maximum binding energy. The Temkin isotherm has been used in the linear form as follows:
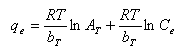 | (6) |
3.8. Biosorption Kinetics
- Parameters from two kinetic models, pseudo first-order and pseudo second-order were fit to experimental data to examine the biosorption kinetics of Cr (VI) uptake onto walnut shell powder.
3.8.1. Pseudo First-order Kinetics
- The pseudo-first order equation of Lagergren[35] is generally expressed as follows:
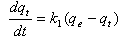 | (7) |
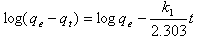 | (8) |
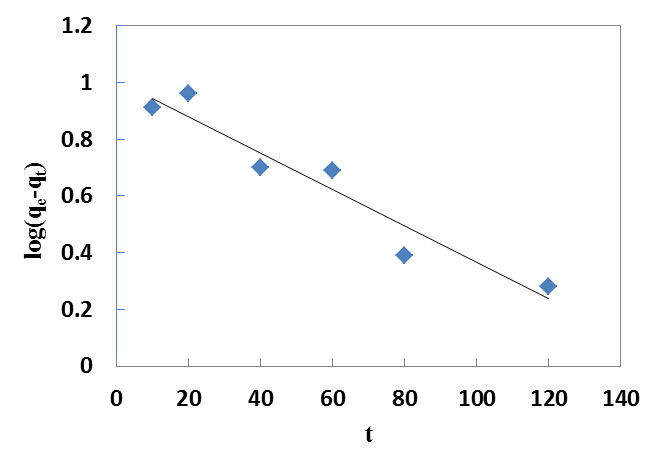 | Figure 5. Pseudo-first order kinetics for Cr(VI) onto walnut shell powder |
3.8.2. Pseudo-second Order Kinetics
- The pseudo second-order rate expression, which has been applied for analyzing chemisorption kinetics rate, is expressed as:
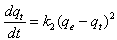 | (9) |
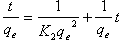 | (10) |
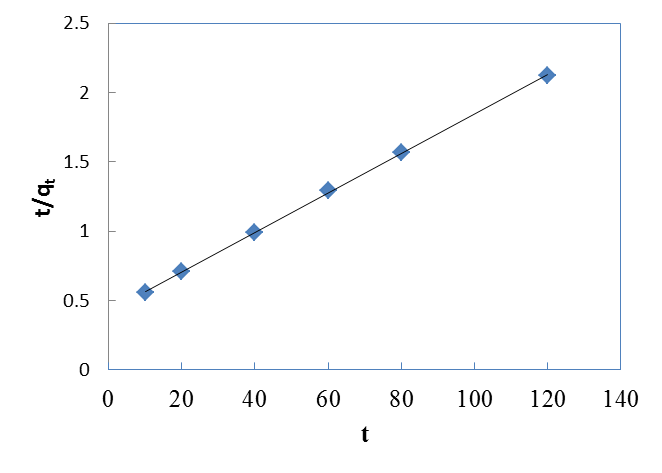 | Figure 6. Pseudo-second order kinetics for Cr(VI) ions onto walnut shell powder |
| |||||||||||||||||||||
3.9. Thermodynamic Parameters
- In order to describe thermodynamic behaviour of the biosorption of Cr(VI) ions onto walnut shell powder, thermodynamic parameters including the change in free energy (∆G◦), enthalpy (∆H◦) and entropy (∆S◦) were calculated from following equations[36-38]:
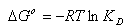 | (11) |
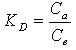 | (12) |
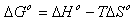 | (13) |
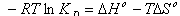 | (14) |
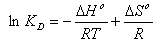 | (15) |
|
3.10. Treatment of Electroplating Wastewater
- The application of Cr(VI) ions biosorption onto walnut shell powder on electroplating wastewater, collected from an electroplating unit of chromium. The main characteristic of the effluent samples before and after the biosorbtion process are listed in Table 5.
|
3.11. Comparison of Walnut Shell Powder with Other Biosorbents
|
4. Conclusions
- In this work, we have studied the biosorption of Cr(VI) ions by walnut shell powder under various conditions. The pH has much effect on the biosorption of Cr(VI) ions from aqueous solutions and electroplating wastewater. The kinetic results provided the best correlation of the experimental data of biosorption of Cr(VI) ions onto walnut shell powder by pseudo second-order equation. The biosorption isotherms could well be fitted by the Langmuir model. The negative values of ∆Go and positive value of ∆Ho revealed that the biosorption process was spontaneous and endothermic. Biosorption process was successfully applied to the treatment of an electroplating wastewater sample, where the concentration of chromium (VI) ions, organic materials and COD were effectively reduced. According to the sorption capacity, walnut shell powder considered as an effective, low cost, and environmentally friendly biosorbent for the removal of Cr(VI) ions from aqueous solution and electroplating wastewater.
ACKNOWLEDGEMENTS
- Authors are thankful for Royal Scientific Society, Jordan University and Baghdad University for providing the necessary facilities to carry out this work.
References
| [1] | Volesky, B., "Detoxification of metal bearing effluents: Biosorption for next century", Elsevier, Hydrometallurgy, vol. 59, pp. 203-216, 2001. |
| [2] | Kadirvelu, K., Thamaraiselvi, K., Namasivayam, C., "Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste", Elsevier, Bioresour. Technol., vol. 76, pp. 63-65, 2001. |
| [3] | Gupta, V.K., Shrivastava, A.K., Neeraj, J., "Biosorption of Chromium (VI) from aqueous solutions by green algae Spirogyra species", Elsevier, Water Res., vol. 35, pp. 4079-4085, 2001. |
| [4] | Kapoor, A., Viraraghavan, T., "Fungal biosorption-an alternative treatment option for heavy metal bearing wastewaters: a review", Elsevier, Bioresour. Technol., vol. 53, pp. 195-206, 1995. |
| [5] | Kumar, R., Bishnoi, N.R., Garima, K.B., "Biosorption of chromium(VI)from aqueous solution and electroplating wastewater using fungal biomass", Elsevier, Chem. Eng. J., vol. 135, pp. 202-208, 2008. |
| [6] | Bishnol, N.R., Kumar R., Bishnoi, K., "Biosorption of Cr(VI) with Triochoderma viride immobilized fungal biomass and cell free Ca-alginate beads", CSIR Publisher, India, Indian J. Expert. Biology, vol. 45, pp. 657-664, 2007. |
| [7] | Marandi, R., Biosorption of hexavalent chromium from aqueous solution by dead fungal biomass of Phanerochaete crysosporium: Batch and fixed bed studies", Wiely, Canadian J. Chem. Eng. & Technol., 2, 8-22. vol. 2, pp. 8-22, 2011. |
| [8] | Wang, X.S., Li, Z.Z., Tan, .S.R., "Removal of Cr(VI) from aqueous solution using walnut hull" Elsevier, J. Environ. Manag., vol. 90, pp. 721-729, 2009. |
| [9] | Sahranavard, M., Ahmadpour, A., Doosti, M.R., "Biosorption of hexavalent chromium ions from aqueous solutions using Almond green hull as a low-cost biosorbent", Elsevier, European J. Sci. Res., vol. 58, pp. 392-400, 2011. |
| [10] | Jain, M., Gang, V.K., Kadirvelu, I., "Chromium(VI) removal from aqueous system using Helianthus annuus (sunflower) stem waste". Elsevier, J. Hazard. Mater., vol. 162, pp. 365-372, 2009. |
| [11] | Park D, Lim S-R, Yun Y-S, Park J.M., "Development of a new Cr(VI)- biosorbent from agricultural biowaste, Elsevier, Bioresour. Technol.,vol. 99, pp. 8810-8818, 2008. |
| [12] | Levankumar, L., Muthukumaran, V., Gobinth, M.B., "Batch adsorption and kinetics of chromium (VI) from aqueous solutions by Ocimum americanum L. seed pods" Elsevier, J. Hazard. Mater., vol. 161, pp. 709-713, 2009. |
| [13] | Suksabye, P., Thiravetyan, I., "Cr(VI) adsorption from electroplating plating wastewater by chemically modified coir pith", Wiley, J. Environ. Manag., vol. 102, pp. 1-8, 2012. |
| [14] | Idris, S., Lyake, Y.A., Dauda, B.E.N., Ndamtso, M.M., Umar, M.T., "Kinetic study of utilizing groundnut shell as an adsorbent in removing chromium and nickel from dye effluent", Science domain International, Amer. Chem. Sci. J., vol. 2, pp. 12-24, 2012 |
| [15] | Acharya, J., Sahu, J.N., Sahoo, B.K., Mohanty, C.R., Meikap, B.C., "Removal of chromium (VI) from wastewater by activated carbon developed from Tamarind wood activated with zinc chloride", Elsevier, Chem. Eng. J., vol. 150, pp. 25-39, 2009. |
| [16] | Blazquez, G., Hernainz, F., Calero, M., Martin-Lara, M.A., and Tenorio, G., “The effect of pH on the biosorption of Cr (III) and Cr (VI) with olive stone” ; Elsevier, Chem. Eng. J., vol. 148, pp. 473-478, 2009. |
| [17] | Chand, R., Narimura, K., Kawakita, H., Ohto, K. "Grape waste as a biosorbent for removing Cr (III) from aqueous solution", Elsevier, J. Hazard. Mater., vol. 163, pp. 245-250, 2009. |
| [18] | Cimino, G., Passerini, A., and Toscano, G., "Removal of toxic cations and Cr (VI) from aqueous solution by hazelnut shell", Elsevier, Water Res. , vol. 34, pp. 2955-2962, 2000. |
| [19] | Pehlivan, E., Altun, T., "Biosorption of chromium (VI) ion from aqueous solution using walnut, hazelnut and almond shell”, Elsevier, J. Hazard. Mater., vol. 155, pp. 378-384, 2008. |
| [20] | Moussavi, G., Barikbin, B., "Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass", Elsevier, Chem. Eng. J., vol. 162, pp. 893-900, 2010. |
| [21] | Banasal M., Singh D. V.K., Garg V.K., "A comparative study for the removal of hexavalent chromium from aqueous solution by agriculture wastes’ carbons”, Elsevier, J. Hazard. Mater., vol. 171, pp. 83-92, 2009. |
| [22] | Gao, H., Liu, Y., Zeng, G., Xu, W., Li, T., Xia, W., "Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste-rice straw", J. Hazard. Mater., vol. 150, pp. 446-452, 2008. |
| [23] | Prasad, A.G., Abdullah, M.A., "Biosorption of Cr(VI) from synthetic wastewater using the fruit shell of gulmohar (Delonix regia): Application to electroplating wastewater", NC State University, Bioresources, vol. 5, pp. 838-853, 2010. |
| [24] | Tan, W.T, Ooi, S.T., Lee, C.K., "Removal of chromium from solution by coconut husk", Taylor & Francis, Environ. Technol., vol. 14, pp. 277-282, 1993. |
| [25] | Ahalya, N., Kanamadi, R.D., Ramachandr, A., "Biosorption of chromium (VI) from aqueous solutions by the husk of Bengal gram" Universidad Catolica de Valparaiso, Chile, Electronic J. Biotechnology, vol. 8, pp. 258-264, 2005. |
| [26] | Sarin, V., Pant, K.K., "Removal of chromium from industrial waste by using eucalyptus bark", Elsevier, Bioresour. Technol. vol. 97, pp. 15-20, 2006. (2006). |
| [27] | Garg, U.K., Kaur M.P., Garg, V.K., Sud, D., "Removal of hexavalent chromium from aqueous solution by agricultural waste biomass", Elsevier, J. Hazard. Mater., vol. 140, pp. 60-68, 2007. |
| [28] | Dakiky, M., Khamis, M., Manassra, A., Mereb, M., "Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents", Elsevier, Adv. Environ. Res., vol. 6, pp. 533-540, 2006. |
| [29] | Sharma, D.C., Forster, C.F., "A preliminary examination into the adsorption of hexavalent chromium using low-cost adsorbents", Elsevier, Bioresour. Technol., vol. 47, pp. 257-264, 1994. |
| [30] | Sharma, D.C., Forster, C.F., "The treatment of chromium wastewater using the sorptive potential of leaf mould", Elsevier, Bioresour. Technol. vol. 49, pp. 31-40, 1994. (2006). |
| [31] | Malkoc, E., Nuhoglu, Y., Dundar, M., "Adsorption of chromium(VI) on pomace-An olive oil industry waste: Batch and column studies", Elsevier, J. Hazard. Mater., vol. 138, pp. 142-151, 2006. |
| [32] | APHA: Standard methods for the examination of water and wastewater. 21st Edn. Washington, D.C. (2005). |
| [33] | Langmuir I., "The adsorption of gases on plane surfaces of glass, mica and platinum", American Chemical Society, J. Amer. Chem. Soc., vol. 40, pp. 1361-1403, 1918. |
| [34] | Freundlich H., and Hellen W., "The adsorption of Cis- and Trans-Azobenzen", American Chemical Society, J. Amer. Chem. Soc., vol. 61, pp. 2228-2230, 1993. |
| [35] | Aharoni, A., Ungarish, M. "Kinetics of activated chemisorption Part 2. Theoretical models", RSC Publishing, J. Chem. Soc. Faraday Trans., vol. 73, pp. 456-464, 1997. |
| [36] | Lagergren S., "About the theory of so-called adsorption of soluble substances". K. Sven. Vetenskapsakad Handl, WIKIPEDIA, 24, 1–39. vol. 24, pp. 1-39, 1898. |
| [37] | Singha, B., Das, S.K.,"Biosorption of Cr(VI) from aqueous solutions: Kinetic equilibrium thermodynamic and desorption studies", Elsevier, Colloids Surf. B: Biointerfaces, vol. 84, pp. 221-232, 2011. |
| [38] | Qu, R., Zhang, Y., Sun, C., Wang, C., Ji, C., Chen, H., Yin, P., "Adsorption of Hg(II) from different routes: Kinetic, thermodynamics and isotherms", American Chemical Society, J. Chem. Eng. Data, vol. 55, pp. 1496-1504, 2010. |
| [39] | Uluoz, O.D., Sari, A., Tuzen, M. "Biosorption of antimony from aqueous solutions by lichen (Physcia tribacia)", Elsevier, Chem. Eng. J., vol. 163, pp. 380-388, 2010. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML