-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2012; 2(5): 137-141
doi: 10.5923/j.ajee.20120205.04
Acid Rain and Its Effects on the Lakes of Fars County in Iran
Sohrab Abdollahi
Department of Chemistry, Payame Noor University, I. R. of Iran, PO BOX 19395-3697, Tehran , Iran
Correspondence to: Sohrab Abdollahi , Department of Chemistry, Payame Noor University, I. R. of Iran, PO BOX 19395-3697, Tehran , Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
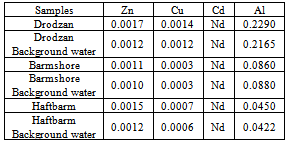
One of the main environmental issue these days is acid rain and its effects on the human and environment. Acid rain effects are more dominant in countries such as United States, Canada, and Europe due to acidic nature of soils in some parts of their lands as well as heavy pollution resulted from vast industrial activities which are conducted through these countries. Contrary to these facts, Iran situation regarding acid rain is totally different and in spite of high pollution in cities and industrial areas, the water of lakes and streams are not acidic. Data collected in this research show that the pH and alkalinity of the lake water and soils are almost high. Some of the lakes in Fars County are dried and the rest are not in normal situation. Lakes of Barmshoor, Droodzan, and Haftbarm have pH around 7.93 -8.07 and alkalinity around 186 to 220 mg/L CaCO3. The soils around the lakes have pH in the range of 7.69-7.89 and alkalinity 208-235 mg/L CaCO3. Therefore both the soil and the water have high alkaline buffer capacity to resist acid rain because; most part of the Fars County consist of calcite, dolomite and some alkaline salts. Pollution load indexes for Al, Zn and Cu for both lake water and related soils are close to one (1.063-1.54) which means no considerable metal pollutions are created by acid rain in Fars County. In fact, high pH and alkalinity of the water and soil make metal salts mostly insoluble and limit the availability of the free metals. The pH changes of rain water show gradual increase of pH during raining. If the sample of rain water is left alone, its pH decreases by residence time.
Keywords: Acid Rain, Soil Alkalinity, Lakes Acidity, Pollution Load Index
Article Outline
1. Introduction
- Acid rain describes any form of precipitation with high levels of nitric and sulfuric acid. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth[1-4].Most acid rain falls because of human activities. The biggest cause of acid rain is the burning of fossil fuels by coal-burning power plants, industries and automobiles. Free CO2, and gases resulted from human burn fossil fuels such as sulfur dioxide, SO2, and nitrogen oxides, NOx are released into the air. These chemical gases react with water, oxygen and some other compound to form a weak solution of carbonic, sulfuric and nitric acid. These acidic particles may spread by wind across the atmosphere and over hundreds of miles. When acid rain precipitate on the Earth, it enters in water streams, lakes and other form of water systems and may sinks into the soil[5,6]. Acid rain have many natural and ecological effects[7]. However its impacts on lakes, water streams and wetlands are greater than others. The main adverse effect of acid rain is that it can wash away the soil aluminium into lakes, and streams. As a result, dissolved aluminium cation, makes lake water and stream toxic and dangerous for crayfish, clams, fish and other aquatic animals[8-10].Increasing amounts of acids can "mobilize" aluminium ions which are normally present in an insoluble non-toxic form of aluminium hydroxide. It appears that when the soil pH dips to 5 or lower, aluminium ions are dissolved into the water and become toxic to plants[11]. Aluminium ions cause a stunting of the root growth and prevent the roots from taking up calcium. The result may be the overall slowing of the growth of the entire tree[12].Lower soil pH and aluminium mobilization can reduce populations of soil microorganisms[13]. Soil bacteria have the job of breaking down the dead and decaying leaves and other debris on the forest floor. The effect of this action is to release nutrients such as calcium, magnesium, phosphate, nitrate, and others. Low pH and high aluminium ion concentrations inhibit this process. Higher amounts of acids can mobilize other toxic metals from the insoluble to the soluble ion forms in the same fashion as aluminium. The toxic metals include lead, mercury, zinc, copper, cadmium, chromium, manganese, and vanadium[14,15].These may all contribute to slow the growth of a tree. In addition, these combination of toxic metals may also adversely affect the growth of soil bacteria, mosses, algae, fungi, and earthworms. That soil may neutralize some or all of the acidity of the acid rainwater. This ability is called buffering capacity, and without it, soils become more acidic[16-18]. Differences in soil buffering capacity are an important reason why some areas that receive acid rain show a lot of damage, while other areas that receive about the same amount of acid rain do not appear to be harmed at all.Researches about acid rain in Iran especially Fars County are very limited in spite of high pollution in cities and industrial centers. Therefore, this study regarding acid rain, lakes of Fars County and soil alkalinity, in fact is the starting steps to illustrate how these factors may affect the environment of Iran. In previous studies, measurements were very simple, superficial and several pH measurements or alkalinity were used as criteria for showing acid rain reality. However this research is more complex and has gone further to show acid rain reality in detail for Fars County.
2. Experimental
2.1. Soil and Water Sampling
- Water samples were collected from middle of lakes. Bottles of pet, sterilized with 60 ppm chlorine were used for water samples. Soil samples were taken from area around lakes located in 2 km from the lake. Soil samples were taken within 30 cm dept. The method of composite sample, i.e., a single sample consisting of the composite of several randomly selected soil cores were used in these experiments. Samples were taken to laboratory no more than 6 hr delay.
2.2. Drying, Crashing and Sieving
- The soil samples were air dried, and the large stone and gravel were removed before crashing process. Thorough mixing requires that the sample be crushed and ground to particles of uniform size. After drying the sample, clods and large aggregates were crushed and mixed. Then the crushed material was further ground to pass 2 mm sieve. The purpose of grinding is to reduce heterogeneity and to provide maximum surface area for physical and chemical reactions. For crashing, pestle and mortal were used.
2.3. water and Soil pH Measurement
- All pH measurements carried out by Metrohm 827 pH meter. The pH meter was calibrated over 7 to 9 range using the standard buffer of 7 and 9. weigh 5 g of sieved and dried soil into a 100 ml beaker. Add 5 mL distilled or deionized water to the sample. Stir vigorously for 15 seconds and let stand for 30 minutes. Then place electrodes in the slurry, swirl carefully, and read the pH immediately. Ensure that the electrode tips are in the slurry and not in the overlying solution.
2.4. Determination of Al, Cu and Zn in Soil
- The instruction of Lindsay and Norvell, (1978) DTPA (diethylenetriaminepentaacetic acid) or DTPA extraction method[19] was used for measurement of these metals in soil samples.
2.4.1. Diethylenetriaminepentaacetic Acid (DTPA) Solution
- Weigh 19.67 g DTPA, with constant stirring, dissolve in 1 liter of distilled water. Weigh separately 149.2 g TEA (triethanol amine) and 14.7 g CaCl2.2H2O, and dissolve in 1 liter of distilled water. Pour under constant stirring the DTPA solution to the TEA-CaCl2.2H2O mixture. After the DTPA has dissolved completely, dilute the solution to 9 liters. Adjust the pH to 7.3 with 1:1 HCl (approximately 42 ml required), and make up the volume to 10 liters with distilled water.
2.4.2. Standard Solutions of Fe, Zn, Cu and Mn
- Stock standard solutions containing 1000 mg/l of the metals.
2.4.3. Preparation of Soil Extract
- 1. Weigh 5 g of air-dried soil (<2 mm) in a 100 ml polyethylene centrifuge tube, add 20 ml DTPA solution, and shake for 30 minutes on a mechanical shaker.2. Centrifuge, and decant into a sample bottle fitted with funnel and filter paper.3. If needed, dilute the extract so that the reading is in the linear working range of the atomic absorption spectrophotometer.
2.4.4. Procedure
- 1. Prepare an intermediate standard solution by pipetting 10 ml from the 1000 μg/ml Stock solution of the analyte into a 200 ml volumetric flask, and dilute to the volume with DTPA solution.2. Prepare standard solutions in the working range, like 0, 1, 2, 5, 10 μg/ml of the trace metal. Always dilute with the DTPA solution.3. Now follow the step by step procedure given in the instruction manual to optimize the working condition of the instrument.4. Measure the signals from the series of working standards of known concentration, and plot the analytical signals (the instrument or detector response) as a function of analyte concentration.
2.5. Pollution Load Index (PLI)
- Pollution load index (PLI), for a particular site, has been evaluated following the method proposed by Tomilson et al. (1980). This parameter is expressed as: PLI = (CF1x CF2 x CF3 x ……….. x CF)1/n where, n is the number of metals (4 in the present study) and CF is the contamination factor. The contamination factor can be calculated from the following relation:
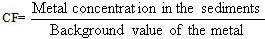
3. Results and Discussion
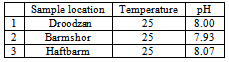
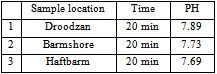
- In Fars county, there are eight main lakes located at different regions. Unfortunately, due to drought, five of the lakes are dried. Lakes of Droodzan, Haftbarm and Barmshor have some water left but they are gradually subjected to water decreasing process. Measurement of water pH for these lakes shows that their waters are alkaline as is shown in Table 1. The pH of the soil slurries are also alkaline Table 2. These facts strongly confirm that the buffer capacity of the soils with respect to acid rain is high.
|
|
|
 | Figure 1. Variation in pH vs. raining duration in Shiraz city |
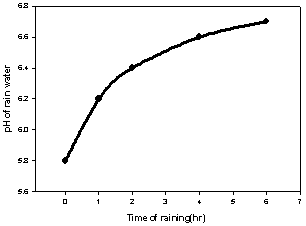
 | Figure 2. Determination of pH of the rain vs. raining duration |
|
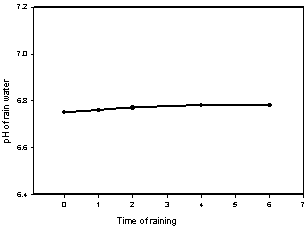
 | Figure 3. Relationship between pH and alkalinity of selected lake water |
|
4. Conclusions
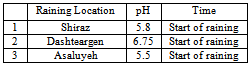
- The main conclusion derived from this research is that the lakes in Fars County are protected by the alkalinity of the soils in the region. Therefore, the acid rain can not be any kind of threat to environmental aspects of the lakes in the near future. In spit of this fact, the drought is going to affect drastically on the environmental issues of the lakes both economically and naturally.However, in cities such as Shiraz and areas like Asaluyeh, the pollution is high and the pH of the rains are acidic; in Shiraz and Asaluyeh, pH of the rain is 5.8, and 5.5 respectively. This acidity could have a lot of destructive effects on constructions, factories and some how human health. Acidic fog at winter and spring can have more damages to trees and plants in mountain foots. Measurement of soil's pollution load index regarding Al, Zn, and Cu confirms the high buffer capacity of alkaline soils in Fars County therefore the rain acidity will decreasedrastically when it contacts with these alkaline soils.
ACKNOWLEDGEMENTS
- I would like to acknowledge the University of Payame Noor of Lamerd, for their financial support and the opportunities that were provided for our research. I sincerely thank Fatemeh Mostaghni and Saeid Zahmatkesh for correction and editing this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML