-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2012; 2(5): 123-127
doi: 10.5923/j.ajee.20120205.02
Biosorption of Cadmium (II) from Aqueous Solutions by Prunus Avium Leaves
Nidá M. Salem 1, Ahlam M. Farhan 2, Akl M. Awwad 3
1Faculty of Agriculture, Jordan University, Amman, Jordan
2Department of Chemistry, College of Science for women, University of Baghdad, Baghdad, Iraq
3Royal Scientific Society, Princess Sumaya University for Technology, El Hassan Science City, Amman, Jordan
Correspondence to: Akl M. Awwad , Royal Scientific Society, Princess Sumaya University for Technology, El Hassan Science City, Amman, Jordan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A new biosorbent from Prunus avium (sweet cherry) leaves was used to remove cadmium(II) from aqueous solutions. The biosorption of cadmium(II) was found to be dependent on solution pH, initial metal ion concentrations, biosorbent dose, and contact time. The experimental equilibrium biosorption data were analyzed by two widely used two-parameters, Langmuir and Freundlich isotherm models. The Langmuir model gave a better fit than the Freundlich model. The kinetic studies indicated that the biosorption process of the cadmium ions followed well pseudo-second-order model. It was concluded that Prunus avium leaves powder can be used as an effective, low cost, and environmentally friendly biosorbent for the removal of Cd(II) ions from aqueous solution.
Keywords: Biosorption, Prunus avium Leaves, Cadmium(II) Ions, BiosorptionIsotherms, Kinetic
Article Outline
1. Introduction
- The major sources of Cd(II) release into the environment by waste streams are electroplating, smelting, alloy manufacturing, pigments, plastic, battery, mining and refining processes. Cadmium is one of the toxic heavy metals, which is regarded as an element of high toxicity. Different methods have been used on cadmium content reduction from water and industrial waste such as chemicalprecipitation, ion exchange, membrane filtration, electrolytic methods, reverse osmosis, solvent extraction, and activated carbon adsorption[1]. These conventional techniques can reduce cadmium ions, but they do not appear to be highly effective due to the limitations in the pH range as well as the high material and operational costs. Different biosorbentmaterials have been investigated for removal of cadmium ions from aqueous solutions such as plant materials like wheat straw[2], agricultural waste biomass[3], rice husk[4], orange peel[5], nut husk[6], sugar beet pulp[7], orange waste[8], coconut shell[9], juniper bark and wood[10] and wheat stem[11].In recent years, considerable attention has been focused on the removal of cadmium ions from aqueous solution using adsorbents derived from low-cost tree leaves such as loquat leaves[12], sidiumguajava eaves[13], maize leaf[14], ulmus leaves[15], Scolymus hispanicus.[16], Ulmus carpinifolia and Fraxinus excelsior tree leaves[17],In the present work, We have studied the potential of cadmium(II) ions biosorption on Prunus avium (sweet cherry) coming from P. avium tree leaves. Results from this study can be used to assess the utility of P. avium leaves powder for cadmium(II) ions removal from water and industrial wastewaters.
2. Materials and Methods
2.1. Adsorbent
- The raw Prunus avium (sweet cherry) leaves werecollected from a local plantation. The leaves were thoroughly rinsed with water to remove dust and soluble materials. Then it was allowed to dry at room temperature. The dried leaves was grounded to a fine powder in a grinding mill (Retsch RM 100) and sieved to get size fraction < 44 µm, and then dried in an oven at 60℃for 24 h.
2.2. Materials
- All the chemicals used were of analytical reagent (AR) grade. Stock solutions of 1000 mg/L of cadmium(II) ionswere prepared from nitrates of cadmium which was purchased from Fluka AG using double distilled water. Desired test solutions of cadmium ions were prepared using appropriate subsequent dilutions of the stock solution. The range of concentrations of cadmium ions prepared from standard solution varies between 10 and 100 mg/L. Before mixing the adsorbent, the pH of each test solution was adjusted to the required value with 0.1 M NaOH or 0.1 M HCl.
2.3. Analysis
- The concentrations of Cd(II) ions in the solutions before and after equilibrium were determined by AAS6300 Atomic absorption spectrometer (Shimadzu, Japan). The pH of the solution was measured with a WTW pH meter using a combined glass electrode. Fourier Transform Infrared Spectroscopy, FTIR (IR Prestige-21, Shimadzu, Japan) was used to identify the different chemical functional groups present in the P. avium leaves powder. FTIR analyses also used to determine the functional groups which are responsible for the cadmium binding with P. avium leaves powder. The analysis was carried out uing KBr and the spectral range varying from 4000 to 400 cm−1.
2.4. BiosorptionExperiments
- Batch biosorption experiments were conducted by mixing biosorbent with cadmium ion solutions with desiredconcentration in 250 mL glass flask. The glass flasks were stoppered during the equilibration period and placed on a temperature controlled shaker at a speed 120 r/min. The effect of pH on the equilibrium biosorption of Cd(II) was investigated by mixing, The amount of biosorption was calculated based on the difference between the initial (Co, mg/L) and final concentration (Ce, mg/L) in every flask, as follows:
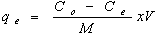 | (1) |
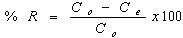 | (2) |
3. Results and Discussion
3.1. FT-IR Analysis
- To investigate the functional groups of P. avium and cadmium loaded on P. avium, a FT-IR study was carried out and the spectra are shown in Figures. 1. and 2. The P. avium leaves display a number of absorption peaks, reflecting their complex nature. A strong and broad peak at 3356cm−1 results due to the stretching of the N–H bond of amino groups and indicative of bonded hydroxyl group of alcohols and phenols. A change in peak position to 3375 cm-1 in the spectrum of the cadmium loaded P. avium, Fig.2, indicates the binding of cadmium with amino and/or hydroxyl groups. The absorption peaks at 2939 cm−1 and 2893cm−1 could be assigned to –CH stretching vibrations of –CH3 and –CH2 functional groups. The shoulder peak in pure P. avium leaves powder at 1701 cm-1 is shifted to lowe frequency 1696 cm-1due to the involvement of carbonyl –CO group from carboxylic acids in the biosorption process of cadmium ions with P. avium leaves powder. The peak at 1635cm−1 indicates the fingerprint region of CO, C–O and O–H groups, which exist as functional groups of P. Avium and shifting of this peak to 1631cm−1, indicated involvement of these groups in cadmium binding. The band at 1377 cm-1 corresponding of C-O stretching was shifted to 1404 cm-1. The region between 1284 and 1000 cm-1 is the fingerprint region, OH, and C-H bending vibration and C-O stretching vibration absorption bands. The intense band at 1029 cm-1 can be assigned to the C-O of alcohols and carboxylic acids. The shift of the peak from 1029 to 1010 cm−1 also suggests the involvement of C–O group in binding Cd(II). The shifts in the absorption peaks generally observed indicate the existence of a cadmium binding process taking place on the surface of the P. avium leaves powder.
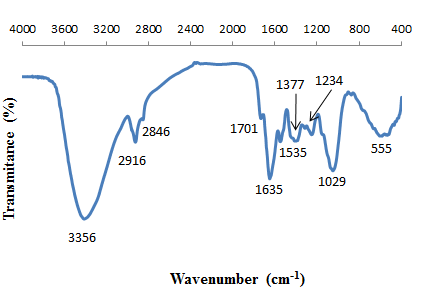 | Figure 1. FTIR of P. avium leaves powder |
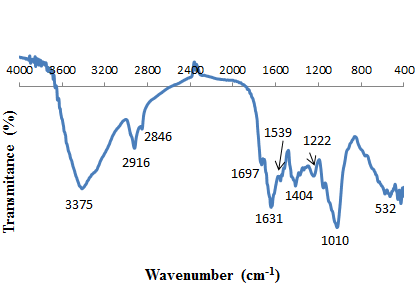 | Figure 2. FTIR of P. avium leaves powder loaded with cadmium |
3.2. Effect of PH
- The pH has been identified as one of the most important parameter that is effective on metal sorption. It is directly related with competition ability of hydrogen ions with metal ions to active sites on the biosorbent surface. The effect of pH on the biosorption of Cd(II) ions onto P.avium leaves powder was studied at pH 1.0–8.0. The maximumbiosorption was observed at pH 6.5 for Cd(II). Therefore, the remaining all biosorption experiments were carried out at this pH values. The biosorption mechanisms on the P.avium leaves powder surface reflect the nature of the physicochemical interaction of the solution. At highly acidic pH (pH < 1.0), the overall surface charge on the active sites became positive and metal cations and protons complete for binding sites on cell wall, which results in lower uptake of metal. The biosorbent surface was more negatively charged as the pH solution increased from 1.0 to 6.0. The functional groups of the P. avium leaves powder were more deprotonated and thus available for the metal ions. Decrease in biosorption yield at higher pH (pH > 6) is not only related the formation of soluble hydroxylated complexes of the metal ions (cadmium ions in the form of Cd(OH)2) but also to the ionized nature of the cell wall surface of the bark powder under the studied pH.
3.3. Effect of Contact Time
- The rate of biosorption is important for designing batch biosorption experiments. Therefore, the effect of contact time on the biosorption of d Cd(II) was investigated. The biosorption yield of Cd(II) increased considerably until the contact time reached 120 min. Further increase in contact time did not enhance the biosorption, so, the optimumcontact time was selected as 120 min for further experiments
3.4. Effect of Adsorbent Dose on Biosorption
- The biosorbent dosage is an important parameter because this determines the capacity of a biosorbent for a given initial concentration. The biosorption efficiency for Cd(II) ions as a function of biosorbent dosage was investigated. The percentage of the metal biosorption steeply increases with the biosorbent loading up to 0.5 g/0.1 L. This result can be explained by the fact that the biosorption sites remain unsaturated during the biosorption reaction whereas the number of sites available for biosorption site increases by increasing the biosorbent dose. The maximumbiosorption 94.44% for Cd(II) was attained at biosorbent dosage, 0.5 g/0.1 L. Therefore, the optimum biosorbent dosage was taken as 0.5 g/0.1 L for further experiments.
3.5. Biosorption Isotherms
- An adsorption isotherm descris the fraction of sorbate molecules that are partitioned between liquid and solid phases at equilibrium. Two isotherm models were tested:
3.5.1. Freundlich isotherm
- The Freundlich isotherm model is the well known earliest relationship describing the adsorption process. This model applies to adsorption on heterogeneous surfaces with the interaction between adsorbed molecules and the application of the Freundlich equation also suggests that sorption energy exponentially decreases on completion of the sorption centers of an adsorbent. This isotherm is an empirical equation and can be employed to describe heterogeneous systems and is expressed as follows in linear form[18]:
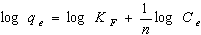 | (3) |
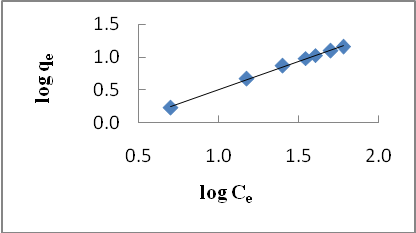 | Figure 3. Freundlich isotherm for Cd (II) biosorption onto P. avium leaves powder at 303 K |
3.5.2. Langmuir isotherm
- The Langmuir isotherm assumes monolayer adsorption on a uniform surface with a finite number of adsorption sites[19]. Once a site is filled, no further sorption can take place at that site. As such the surface will eventually reach a saturation point where the maximum adsorption of the surface will be achieved. The linear form of the Langmuir isotherm model is described as:
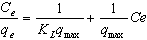 | (4) |
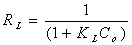 | (5) |
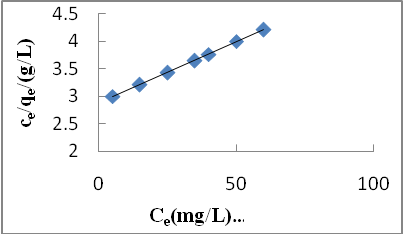 | Figure 4. Langmuir isotherm for Cd (II) biosorption onto P. avium leaves powder at 303 K |
| ||||||||||||||||||||||||
3.6. Biosorption Kinetics
- Parameters from two kinetic models, pseudo first-order and pseudo second-order were fit to experimental data to examine the biosorption kinetics of cadmium (II) uptake by P. avium leaves powder.
3.6.1. Pseudo First-Order Kinetics
- The pseudo-first order equation of Lagergren[20] is generally expressed as follows:
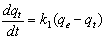 | (6) |
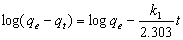 | (7) |
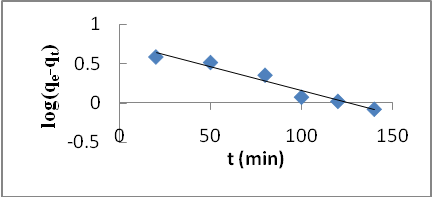 | Figure 5. Pseudo-first order kinetic model for Cd (II) biosorption onto P. avium leaves powder at 303 K |
3.6.2. Pseudo-Second Order Kinetics
- The pseudo second-order rate expression, which has been applied for analyzing chemisorption kinetics rate[20] is expressed as:
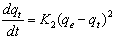 | (8) |
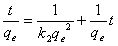 | (9) |
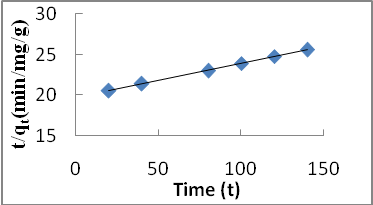 | Figure 6. Pseudo-second order kinetic model for Cd (II) biosorption onto P. avium leaves powder at 303 K |
| |||||||||||||||||||||
4. Conclusions
- The potential of P. avium leaves powder for the removal of Cd(II) ions from aqueous solutions and wastewater was dependent on biosorption process such as pH, initial metal ions concentration, biosorbent dose, and contact time,. The Langmuir and Freundlich biosorption isotherms weredemonstrated to provide best correlation for the biosorption of Cd(II) ions onto P. avium leaves powder. The kinetic results provided the best correlation of the experimental data of biosorption of cadmium ions onto P. avium leaves powder by pseudo second-order equation. it can be concluded that since the P. avium leaves are an easily, locally available, low-cost adsorbent and has a considerable high biosorption capacity , it may be treated as an alternative adsorbent for treatment of wastewater containing cadmium (II) ions.
ACKNOWLEDGMENTS
- Authors are thankful for Royal Scientific Society, Jordan University and Baghdad University for providing the necessary facilities to carry out this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML