-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
p-ISSN: 2166-4633 e-ISSN: 2166-465X
2012; 2(5): 114-122
doi: 10.5923/j.ajee.20120205.01
Greenhouse Experimental Methods Towards in-situ Burial and Restoration of Contaminated Sites in Submerged Wetlands
Alice Benzecry
Fairleigh Dickinson University, School of Natural Sciences, 1000 River Road Teaneck, New Jersey, 07666, USA
Correspondence to: Alice Benzecry , Fairleigh Dickinson University, School of Natural Sciences, 1000 River Road Teaneck, New Jersey, 07666, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
As a result of commercial and industrial activities conducted in the absence of environmental regulations and enforcement in the past, sediments contaminated by organic compounds, heavy metals, and other potentially toxic chemicals have accumulated in many of the world’s deepwater and wetland environments. These sediment-borne contaminants can eventually become incorporated into aquatic food webs and adversely affect ecological receptors like benthic organisms and fish, and ultimately pose a risk to human health. This laboratory research tested a commercial product AquaBlokTM (patented, composite-aggregate technology comprised of a solid core, an outer layer of clay material, and polymers) as an in-situ capping technology that could be used to remediate and/or manage contaminated sediments in the New Jersey Hackensack Meadowlands, a superfund site. In a greenhouse setting, tubs containing representative Meadowland marsh soil and water were capped with AquaBlok. This research not only examined the potential use of this product as an in-situ capping material and possible substrate for flora colonization, but also examined the improvements of the same patented, clay mineral-based composite aggregate technology (SubmerSeedTM) as an alternative to traditional means of wetland plant propagation. At the end of a two-year period, both the sediment/cap and vegetation plant tissues were examined for metallic contaminants (including Cd, Cr, Cu, Pb, Hg, and Zn). Overall, capping provided a less contaminated substrate. Results indicated that AquaBlok cap alone did not allow contaminants in the sediment below to breakthrough. Nevertheless, vegetation colonization was restricted to a limited number of plant species. Furthermore, plants growing in AquaBlok were less robust with lower dry weight and smaller root system than plants growing in uncapped sediments despite the fact that their tissue contained smaller amounts of metallic contaminants. The improvements of the clay mineral-based composite aggregate technology (SubmerSeeds) as an alternative to traditional means of plant propagation worked very well in successfully delivering aquatic plant seeds into permanently inundated conditions.
Keywords: Heavy Metals Contamination, In Situ Capping Material, Aquablok, Wetland Vegetation
Article Outline
1. Introduction
- As a result of commercial and industrial activities conducted in the absence of environmental regulations and enforcement in the past, sediments contaminated by organic compounds, heavy metals, and other potentially toxic chemicals have accumulated in large quantities in the New Jersey Hackensack Meadowlands[1]. The contamination of marine and freshwater sediments with organic and inorganic pollutants has become a worldwide problem with implications for human and ecological health[2]. The need for remediation or management of contaminated sediments has become increasingly evident Contaminated sediments can be managed in various ways: They can be removed by dredging and treated ex-situ, as appropriate, prior to disposal. The sediments can also be managed in-situ by capping, or treatment by biological or chemical means.Among these techniques, treatment of contaminated sediment sites with in-situ caps has become an established practice that can provide advantages over other alternative methods [3]. Advantages of in-situ capping relative to sedimentremoval include minimal environmental and habitat disturbance, minimal sediment exposure and handling, minimal release of volatile organics and elimination of the need for disposal facilities, resulting in lower remediation costs. In addition, in-situ sediment caps are relatively easy to construct, repair or replace as part of ongoing operation and maintenance.Clean sand has traditionally been employed as capping material and remains a large component of many field-scale capping applications. Sand-based caps have the potential to delay contaminant breakthrough when diffusive transport dominates[4-5], but eventual contaminant breakthrough remains a source of concern. Additionally, traditional sand caps are less effective at sites where groundwater seepage or mobile contaminants are present[4].Recent research studies have focused on in-situsequestration[6-7], in-situ transformation[8-9] and the development of active caps that incorporate reactive and/or absorptive constituents designed to reduce contaminant andbioavailability[6],[10-14]. Ideally, active caps eliminate the risk of contaminant breakthrough into the overlying water column, and can potentially be implemented as alternative sediment.The objectives of this laboratory research were to: 1) Test the ability of a patented “active cap” i.e. AquaBlok (AB) to serve as physical barrier between contaminated sediments and overlying biological receptors. 2) Evaluate the ability of plants to colonize AB as an alternative to sediment. 3) Determine if adding organic matter (2% peat) improved AB’s suitability as a substrate. 4) Evaluate the effectiveness of AB pellet (SubmerSeeds) as an alternative tool for plant propagation in permanently inundated waters.
1.1. Why AquaBlokTM
- AquaBlok (AB)[15] is a commercial patented, clay mineral-based composite aggregate technology comprised of a solid core (typically stone aggregate), an outer layer of clay material (bentonite) and polymers. Bentonite, which is well known and widely used throughout the environmental industry, comprises the primary clay material for typical freshwater product formulations, however, other clay or quasi clay-sized materials such as organic matter or plant seeds (Composite Seeding Technology “SubmerSeedsTM”) can also be incorporated into product formulations as needed. Empirical and preliminary laboratory data from the manufacturing industry indicate that AB[16] not only serves as a physical barrier between sediments and overlying biological receptors but it can also serve as a benthic substrate for flora and fauna colonization[17].Based on a series of EPA reports[18-19], AB has distinct advantages over sand for sediment capping with respect to meeting targeted remedial capping functions. It offers physical and chemical blocking of contaminant pathways to overlying receptor organisms and minimizes adverse impacts to wetland hydrology. Granular materials (e.g., sand) can serve as appropriate and adequate capping materials at many sediment remediation sites[20]. Granular materials are typically inexpensive, often locally available, relatively easy to place and can provide substrate for marsh biota. However, typical granular materials are also relatively easily eroded and permeable to the diffusive and adverting movement of dissolved sediment-borne contaminants[21]. Additionally, sand-based remedial caps are often relatively thick (i.e. on the order of feet rather than inches) in order to adequately overcome these performance limitations. Relatively thick caps may adversely affect waterway navigation at some deepwater sites or adversely impact the hydrology and vegetative communities in wetland ecosystems[22-23]. Finer-grained materials (e.g., clays) are typically more cohesive, less permeable, and more reactive than sands. A relatively thin clay-based cap (i.e. on the order of inches rather than feet) can provide a better capping remedy that is less disruptive of wetland quality and function. Additionally, clay has a higher cation exchange capacity than sand making it more effective in trapping contaminants such as heavy metals. The addition of plants seeds into their formulation - Composite Seeding Technology “SubmerSeedsTM” creates an attractive alternative to traditional means of plant propagation in wetland/aquatic settings, especially when confronted with the challenges of establishing a favourable vegetative community in permanently inundated conditions.
2. Experimental Design
- To study the effectiveness of AquaBlok (AB) and AquaBlok amended with 2% peat moss (ABPM) as in-situ “active” capping material and benthic substrate for wetland biota in a greenhouse setting, a total of six 100 gallon tubs containing Hackensack Meadowlands soil and water were used during a two year period. Wetland vegetation seeds of 28 common regional species (Table 1) were incorporated into the AquaBlok formulation to produce SubmerSeedTM.
3. Initial pre-capping analysis
- Tables 2 and 3 represent the Meadowlands sediments characterizations including percent moisture, ash content (Ash), total organic matter (TOC) and grain sizes. The average percentage moisture of the sediment was 91.1%, ash content varied from 11.9 % to 15.2%, and the average total organic matter content was 86.31% (Table 2).
|
|
|
4. Post-capping analysis
4.1. Plant Growth
- A limited number of species of marsh vegetation were able to germinate in the test substrate; only six of the original 28 species prepared as SubmerSeeds: Zizania aquatica ,Alisma subcordatum , Typha angustifolia, Peltandra virginica, Scirpus validus and Scirpus spp.. Large numbers of seedlings of Peltandra virginica, Scirpus validus and Scirpus spp. germinated but were not able to reach full maturity. Due to the low numbers of established matured representatives of these species, they were not fully considered under this study.During the first growing season, wetland vegetation grew better in uncapped sediments when compared to AquaBlok capped sediments. Zizania aquatica, Alisma subcordatum and Typha angustifolia germinated and developed better in ABPM when compared to AB alone. In addition to the above-mentioned species, Phragmites australis from the local seed/rhizome banks equally colonized both capped and uncapped sediments (Figure 1)Observations of wetland vegetation during the second growing season revealed that plant germination and growth rates in ABPM declined considerably when compared to the AB and Soil treatments (Figure 1).
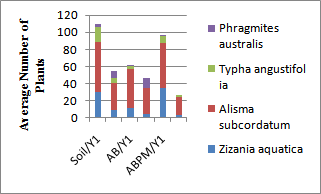 | Figure 1. Average number of plants that germinated and reached maturity at the different treatments; Soil, AB and ABPM. Y1=first growing season, Y2= second growing season |
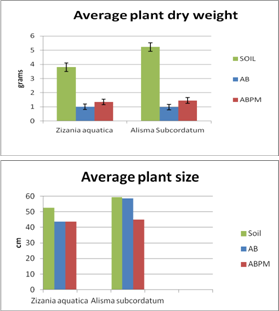 | Figure 2. Average size and dry weight of two plant species representatives growing in different treatments. Soil, AB and ABPM |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
 | Figure 3. plants growing in the different treatments: Soil, AB and ABPM |
 | Figure 4. Top left = roots of Alisma subcordatum growing in soil, ABPM and AB. Top right = same roots after heavy washing to remove the clay. Middle = roots of Typha angustifolia growing in soil, ABPM and AB. Middle right = same roots after heavy washing to remove the clay. Bottom left= roots of Zizania aquatica growing in soil and ABPM |
4.2. Heavy Metals of Concern
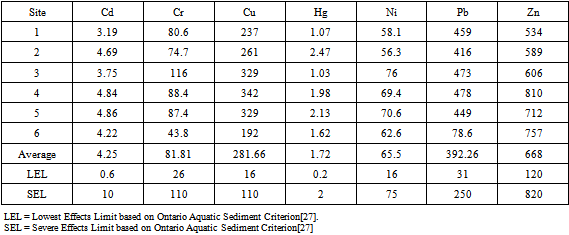
- Heavy metal of concern concentrations in sediment shows treatment related effects (Figure 5). Total metalconcentrations in AquaBlok (AB and ABPM) treated tubs declined significantly after capping from 1496.83 mg/Kg to 330.70 mg/Kg in AB and 315.18 mg/Kg in ABPM. In uncapped tubs (soil), sediment concentrations of Cu (450.67 mg/Kg) and Pb (460.63 mg/Kg) were consistently above their SEL and Hg (1.21 mg/Kg) and N (45.72 mg/Kg) were above LEL when compared to capped sediment tubs. Cd and Hg was above LEL in all tubes, uncapped soil had twice as much Cd and five times more Hg than capped ones. Uncapped sediments had 5 to 6 times more Cr and Cu than capped sediments. Ni quantities above ELE in uncapped sediments were five and one half times higher than capped sediments. Pb levels above SEL in uncapped sediments were also five and one half times higher than capped sediments.
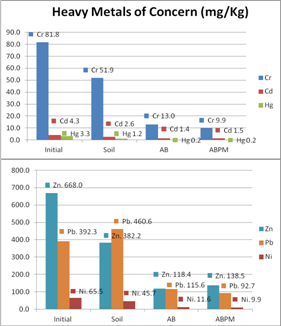 | Figure 5. Amounts of heavy metals of concern variation in sediments at the different experimental treatments (Soil, AB and ABPM) before (initial) and after two vegetation growing seasons |
|
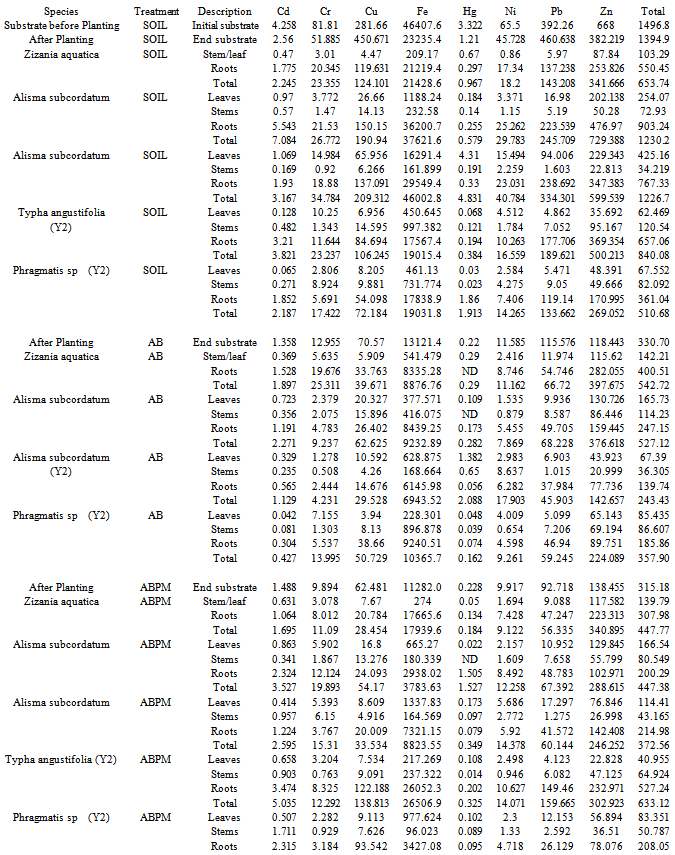
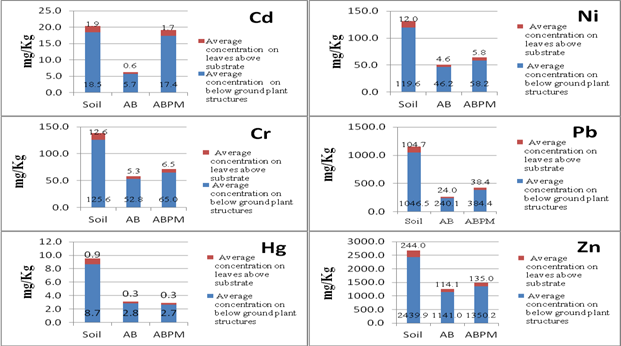 | Figure 6. Amounts of heavy metals of concern (HMOC) in plant tissues from representatives growing in the different experimental sediment treatments (Soil, AB and ABPM) |
5. Conclusions
- Overall, capping provided a less contaminated substrate, 1496.83 mg/Kg total contaminant of concern versus 330.70 mg/Kg in AB and 315.18 mg/Kg in ABPM. The concentrations of metals in the cap itself were much lower than in the sediments they covered. Comparison of collection dates showed no significant increase in heavy metals in the cap over a two-year period indicated that the heavy metals below the cap were not breaking through it. Amending the AquaBlok with peat moss (ABPM) did not significantly affect metal concentrations or plant growth. The initial germination and growth of plants in ABPM was better when compared to AB alone, but it quickly declined after the first growing season. In general, plants growing in AB and ABPM as an alternative sediment source were less robust than plants growing in uncapped sediments control (soil) despite the fact that they have smaller amounts of heavy metals in their tissues. Most of the plants growing in the experimental tubs (soil, AB and ABPM) concentrated higher amounts of heavy metals into their roots and/or underground portion of their stems, between 2.5 and 5 times more than the amounts concentrated in their aerial stems and leave. This is consistent with other research findings such as the works of Raskin et al.[28], Sawidis et al.[29], Bennett et al.[30], and Reboreda and Caçador[31]. In all, the reduction of heavy metals provided by the capping material did not increase the growth or the health of vegetation in contaminated environment. AquaBlok has proven to be an active barrier between contaminants and the biota. Nevertheless, it is not a good substrate for plant colonization despite the addition of organic matter (2% peat moss) to its formulation. Plants growing in AquaBlok capped sediments were less robust with lower dry weight and smaller root systems. The improvements of the clay mineral-based composite aggregate technology (SubmerSeeds) as an alternative to traditional means of plant propagation worked very well in successfully delivering aquatic plant seeds into permanently inundated conditions.
ACKNOWLEDGMENTS
- This research was partially funded by an EPA grant XP 97287404-0 and Fairleigh Dickinson University faculty grant-in-aid.Special thanks to Eduard Konsevick, Michael Pena, Louise Lynch, Kaitlin Le, Sameera Peddada, Anna Fauquet, and the Meadowlands Environmental Research Institute (MERI) lab personal for their help and contribution to this project.
References
| [1] | Langan Engineering and Environmental Services, Inc., 1999. Environmental Assessment Report. Available as Document 1 at www.rerc.rutgers.edu/kearnymarsh/publications.html. |
| [2] | USEPA (US Environmental Protection Agency), 1997. Ecological risk assessment guidance for Superfund: process for designing and conducting ecological risk assessment. U.S. Environmental Protection Agency, Washington, D.C (1997) EPA/540/R-97/006 |
| [3] | D. Reible, , D. Hayes, C. Lue-Hing, J. Patterson, N. Bhowmik, M. Johnson, J. Teal. 2003. Comparison of the long-term risks of removal and in situ management of contaminated sediments in the Fox River. Soil & Sediment Contamination, 12 (3) (2003), pp. 325–344 |
| [4] | J.L. Go, D.J. Lampert, J.A. Stegemann, D.D. Reible. 2009. Predicting contaminant fate and transport in sediment caps: mathematical modeling approaches. Applied Geochemistry., 24 (7) (2009), pp. 1347–1353. |
| [5] | G.J Thoma, D.D. Reible, K.T. Valsaraj, L.J. Thibodeaux. 1993. Efficiency of capping contaminated sediments in situ. 2. Mathematics of diffusion adsorption in the capping layer. Environmental Science & Technology, 27 (12) (1993), pp. 2412–2419. |
| [6] | H. Choi, , S. Agarwal, S.R. Al-Abed. 2009. Adsorption and simultaneous dechlorination of PCBs by GAC impregnated with ZVI/Pd bimetallic particles: mechanistic aspects and reactive capping barrier concept. Environmental Science & Technology, 43 (2) (2009), pp. 488–493 |
| [7] | J.R.Zimmerman, U. Ghosh, R.N. Millward, T.S. Bridges, R.G. Luthy. Addition of carbon sorbents to reduce PCB and PAH bioavailability in marine sediments: physicochemical tests. Environmental Science & Technology, 38 (20) (2004), pp. 5458–5464 |
| [8] | V.Krumins, J.-W. Park, E.-K. Son, L.A. Rodenburg, L.J. Kerkhof, M.M. Häggblom, D.E. Fennell. 2009. PCB dechlorination enhancement in Anacostia River sediment microcosms. Water Research, 43 (18) (2009), pp. 4549–4558 |
| [9] | G.V. Lowry, K.M. Johnson. 2004. Congener specific PCB dechlorination by microscale and nanoscale zero-valent iron in a methanol/water solution. Environmental Science & Technology, 38 (19) (2004), pp. 5208–5216 |
| [10] | S. Hyun, C.T. Jafvert, L.S. Lee, P.S.C. Rao. 2006. Laboratory studies to characterize the efficacy of sand capping a coal tar-contaminated sediment. Chemosphere, 63 (10) (2006), pp. 1621–1631. |
| [11] | P.H. Jacobs, U. Förstner. 1999. Concept of subaqueous capping of contaminated sediments with active barrier systems (ABS) using natural and modified zeolites. Water Research, 33 (9) (1999), pp. 2083–2087. |
| [12] | K.M. McDonough, P. Murphy, J. Olsta, Y.W. Zhu, D. Reible, G.V. Lowry. Development and placement of a sorbent-amended thin layer sediment cap in the Anacostia River. Soil & Sediment Contamination, 16 (3) (2007), pp. 313–322. |
| [13] | P. Murphy, A. Marquette, D. Reible, G.V. Lowry. 2006. Predicting the performance of activated carbon-, coke-, and soil-amended thin layer sediment caps. Journal of Environmental Engineering – ASCE, 132 (7) (2006), pp. 787–794. |
| [14] | D.D. Reible, D. Lampert, D.W. Constant, R.D. Mutch, Y. Zhu. 2007. Active capping demonstration in the Anacostia River, Washington, DC. Remediation, 17 (1) (2007), pp. 39–53. |
| [15] | AquaBlok, Ltd., http://www.aquablokinfo.com/ |
| [16] | J.H. Hull, J.M. Jersak, and C.A. Kasper. 1999. In-Situ Capping of Contaminated Sediments: Comparing the Relative Effectiveness of Sand Versus Clay Mineral-Based Sediment Caps. Unpublished. |
| [17] | J.H. Hull, J.M. Jersak, and H.F. Crowell. 2000. ‘Biofriendly’ Remediation of Impacted Wetlands. In: JR Means and RE Hitchee (eds’) Wetlands and Remediation International Conference Proceedings, pp 229-236. |
| [18] | U.S. EPA Releases SITE Evaluation Report – accessed from http://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P10030OW |
| [19] | Demonstration of the AquaBlok Sediment Capping Technology.http://www.epa.gov/region1/superfund/sites/nyanza/466646.pdf |
| [20] | M.R. Palermo, R.A. Shafer, J.M. Brannon, C.L. Truitt, M.E. Zappi, J.G. Skogerboe, S.A. Adamec, T.C. Sturgis, R. Wade, D. Gunnison, and T.E. Myers. 1989. “Evaluation of Dredged Material Disposal Alternatives for US Navy Homeport at Everett, Washington,” Technical Report, EL-89-1, US Army Engineer Waterways Experiment Station, Vicksburg, MS |
| [21] | M.S. Dortch, L.Z. Hales, J.V. Letter, and W.H. McAnally. 1990. “Methods of Determining the Long Term Fate of Dredged Material for Aquatic Disposal Sites,” Technical Report, D-90-1, U.S. Army Engineer Waterways Experiment Station, Vicksburg, MS. |
| [22] | Hugo Coops and Gerard van der Velde 1995. Seed dispersal, germination and seedling growth of six helophyte species in relation to water-level zonation. Freshwater Biology. Volume 34 Issue 1. pp 1-13 |
| [23] | Kellogg, Chev H. Scott D. Bridgham† and Stacey A. Leicht 2003. Effects of water level, shade and time on germination and growth of freshwater marsh plants along a simulated successional gradien Journal of Ecology Volume 91 pp 274. |
| [24] | American Society for Testing and Materials (ASTM) International. 2007. ASTM D2974-00 Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils. |
| [25] | American Society for Testing and Materials (ASTM) International. 2007. ASTM D422 – 63 Standard Test Method for Particle-Size Analysis of Soils |
| [26] | United States Environmental Protection Agency 2005. Fourth edition. "Test Methods for Evaluating Solid Waste, Physical/Chemical Methods", SW-846. |
| [27] | D. Persaud, Jaagumagi, R., and A. Hayton, 1992. Guidelines for the Protectionand Management of Aquatic Sediment Quality in Ontario. Ontario Ministry of the Environment, Queen's Printer for Ontario. |
| [28] | Ilya Raskin, PBA Nanda Kumar, Slavik Dushenkov, David E Salt. 1994. Bioconcentration of heavymetals by plants. Current Opinion in Biotechnology. Volume 5, Issue 3, pp. 285–290 |
| [29] | T Sawidis, M K Chettri, G A Zachariadis, J A Stratis. 1995. Heavy metals in aquatic plants and sediments from water systems in Macedonia, Greece. Ecotoxicology and Environmental Safety. Volume: 32, Issue: 1, pp. 73-80 |
| [30] | James P. Bennett, Esteban Chiriboga, John Coleman, Donald M. Waller. 2000. Heavy metals in wild rice from northern Wisconsin. Science of The Total Environment, Volume 246, Issues 2–3, pp. 261-269 |
| [31] | R. Reboreda, I. Caçador. 2007. Copper, zinc and lead speciation in salt marsh sediments colonized by Halimione portulacoides and Spartina maritime. Chemosphere, 69-pp. 1655–166. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML