-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
2012; 2(2): 31-34
doi: 10.5923/j.ajee.20120202.05
Production of Xanthan employing Xanthomonas campestris using Sugarcane Molasses
Murugesan A. G. 1, Dhevahi B. 1, Gowdhaman D. 2, Bala Amutha K. 1, Sathesh Prabu C. 1
1Sri Paramakalyani Centre of Excellence in Environmental Sciences, Manonmaniam Sundaranar University, Alwarkurichi - 627 412. Tamil Nadu, India
2Department of Biotechnology, School of Chemical and Biotechnology, Sastra University, Tirumalaisamudram, Thanjavur – 613401, Tamil Nadu, India
Correspondence to: Murugesan A. G. , Sri Paramakalyani Centre of Excellence in Environmental Sciences, Manonmaniam Sundaranar University, Alwarkurichi - 627 412. Tamil Nadu, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Xanthan production by Xanthomonas campestris from pre-treated sugarcane molasses (acidified molasses and acidified aerated molasses) was investigated. The optimization of xanthan yield was done at different pH, temperature, and incubation time for both the pre-treated sugarcane molasses. Maximum yield was achieved in 1% acidified molasses and acidified aerated molasses with 1% yeast extract as nitrogen source for 3 days of incubation time at 30℃. The precipitation of xanthan gum was done after 48 hours and the yield was high in acidified molasses and acidified aerated molasses comparing to acidified sugarcane molasses. Xanthomonas campestris produced xanthan yield of 12.23 g/l using acidified molasses and acidified aerated molasses. This fermentation study has an advantage over some other manufacturing processes with its use of agro industrial wastes as the raw material, allowing the increased xanthan production.
Keywords: Xanthomonas campestris, sugarcane molasses, xanthan, acidified molasses, acidified aerated molasses
Article Outline
1. Introduction
- Xanthan gum is the most commercially accepted microbial extracellular heteropolysaccharide used in oil, food, and pharmaceutical industries as viscosifier, thickener, stabilizer, and emulsifier [1, 2]. The efficient xanthan gum producer is Xanthomonas campestris. Other species such as X. phaseolis, X. malvacearum, X. carotae, X. manihotis and X. jugandlis are known to produce this exopolysaccharide as well. At present the annual production capacity is 5000 tons in India. The demand for xanthan is increasing every year. Research on the biosynthesis process has been recently focused on the biochemistry of the production[3] and the development of several culture methods to improve yields[4], and the search of X. campestris mutants with increased xanthan production [5]. Most studies report the clear dependency between strain used and its xanthan yield and properties[6].The nutritional veracity of X. campestris allows the use of industrial, complex, and synthetic media formulations. The industrial scale production of xanthan is carried out using inexpensive substrates and nutrients. Efficient conversion of carbon source to the desired polysaccharide production requires a high carbon to nitrogen ratio. Sugarcane molasses is the main by product in sugarcane industry having a rich source of nutrients that lead environmental pollution. The volume of effluents varies depending on cane industrial operation and productions, as per IS: 4902-1979, the average volume of effluents production is 1320 l/day. In the present study, xanthan production from the fermentation media containing acidified sugarcane molasses (ASM) and acidified, aerated sugarcane molasses (AASM) employing X. campestris was carried out.
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
- Xanthan producing bacterium, Xanthomonas campestris (MTCC 2286) was procured from the Microbial Type Culture Collection and Gene Bank of Institute of Microbial Technology, Chandigarh, India. X. campestris was maintained in LB sucrose media (sucrose - 1g/l, yeast extract - 0.1g/l, peptone - 0.5g/l, NaCl- 0.3g/l). Flasks containing 50 ml of specified medium were inoculated with a loopful of cells. After 48 h of growth at 30℃, 50 ml of inoculum attained cloudiness. The inoculum was used directly or held at 4℃ until needed.
2.2. Collection and Analysis of Sugarcane Molasses
- The sugarcane molasses was collected from the Government sugar mill, Alanganallur, Madurai District, Tamil Nadu. The physico-chemical characteristics of sugar cane molasses such as color, odor, pH, total suspended solids (TSS), total dissolved solids (TDS), biochemical oxygen demand (BOD), chemical oxygen demand (COD), acidity, total Kjeldahl nitrogen, sulphides, oils and grease were analyzed following the standard methods[7].
2.3. Pre-treatment of Sugarcane Molasses
- Two types of pre-treatment methods were adopted for sugarcane molasses. 200 ml of the sugarcane molasses was acidified with 10 ml of 5 N H2SO4 and it was precipitated at pH 4 and stored (Acidified sugarcane molasses – ASM) and ASM was then aerated by air sparing, 3 ppm for 15 min at 80℃ and stored (Acidified, aerated sugarcane molasses – AASM).
2.4. Optimization of Sugarcane Molasses and Nitrogen Source Concentration
- The optimum concentration of ASM and AASM as sole carbon source and yeast extract as nitrogen source were determined by culturing 5% (v/v) of inoculum in medium (K2HPO4-2g/l, MgSO4-0.1g/l, Tween 80-300 ppm) amended with different concentrations (0.5%,1%, 1.5% and 2%) of ASM, AASM, and yeast extract, incubated at 30℃ for 48 h. Cell growth was monitored by measuring the optical density (OD) at 420 nm (OD420; UV – Visible spectrophotometer- Genesys 2, Spectronic Instruments, NY) at 24 h and 48 h. Dry cell weight (DCW) was also estimated at 48 h[8]. The xanthan produced was recovered from cell-free supernatant by precipitation with two volumes of ice cold isopropanol with addition of 1% (w/v) KCl and dry weight was obtained by freeze-drying of xanthan precipitated according to the methods of Lopez et al.[9].
2.5. Optimization of pH, Temperature and Incubation Period
- Medium for optimization studies (ASM/AASM-10g/l, yeast extract-10g/l, K2HPO4–2g/l, MgSO4-0.1g/l, Tween–80 ppm) was prepared with different pH 6.6, 6.8, 7.0, and 7.2, and 5% (v/v) of 48 h grown culture was inoculated and incubated at 30±2℃. For optimization of temperature, the medium with 5% (v/v) inoculum was incubated at different temperatures such as 26℃, 28℃, 30℃ and 32℃ for 48 h. Xanthan yield, OD and DCW were estimated after 24 and 48 h following the standard procedures. For optimization of incubation period, OD, DCW and xanthan yield under the optimized conditions were estimated at 36, 40, 44, 48 and 52 h.
3. Results and Discussion
- The characteristics of sugarcane molasses taken as the carbon source were analysed and the results were noted. The optimized concentration of ASM and AASM as carbon source was found to be 1% which showed maximum cell biomass and xanthan yield (Figure 1). Various concentrations of yeast extract influenced the growth of organism and xanthan yield (Figure 2). The xanthan yield was high at 1% yeast extract. The best suited temperature for bacterial growth and xanthan production was found to be 30℃ followed by room temperature, 25℃ and 45℃ (Figure 3). The best bacterial growth and xanthan production followed a pH 9> 6.2 > 6.4 > 6.6 > 7 > 7.2 sequence (Figure 4). The xanthan production increased from 24 h to 48 h of growth and declined thereafter. X. campestris produced high xanthan yield of 12.23 and 10.3g/l with 1% AASM and ASM broth respectively under optimized condition.
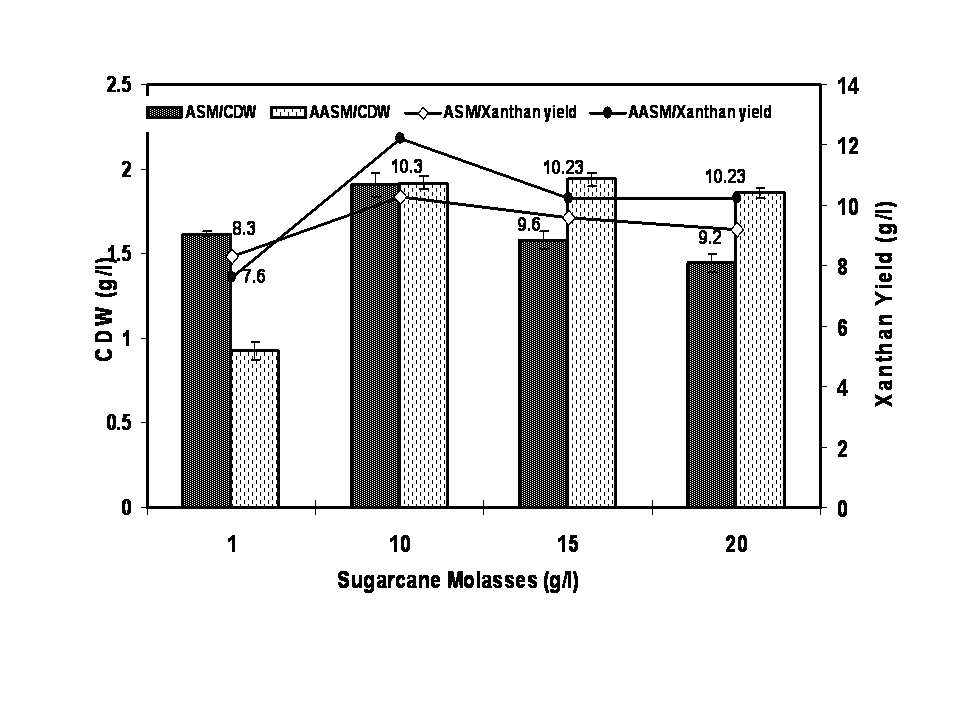 | Figure 1. Optimization of sugar cane molasses as carbon source for xanthan production using Xanthomonas campestris |
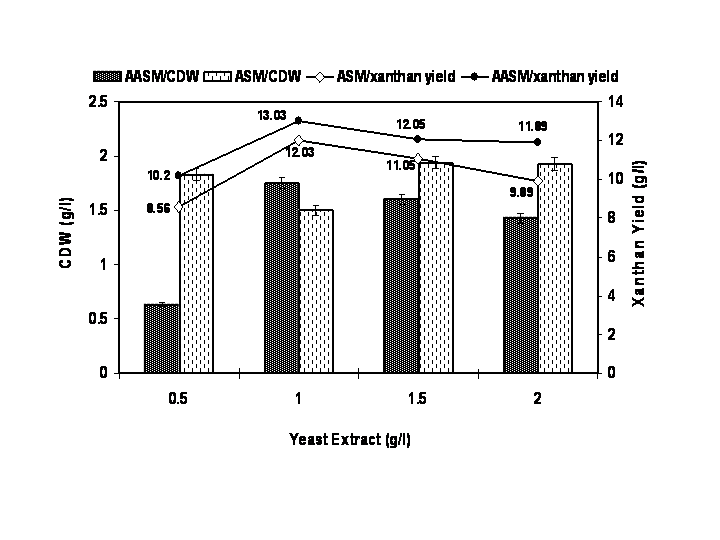 | Figure 2. Optimization of yeast extract as nitrogen source for xanthan production using Xanthomonas campestris |
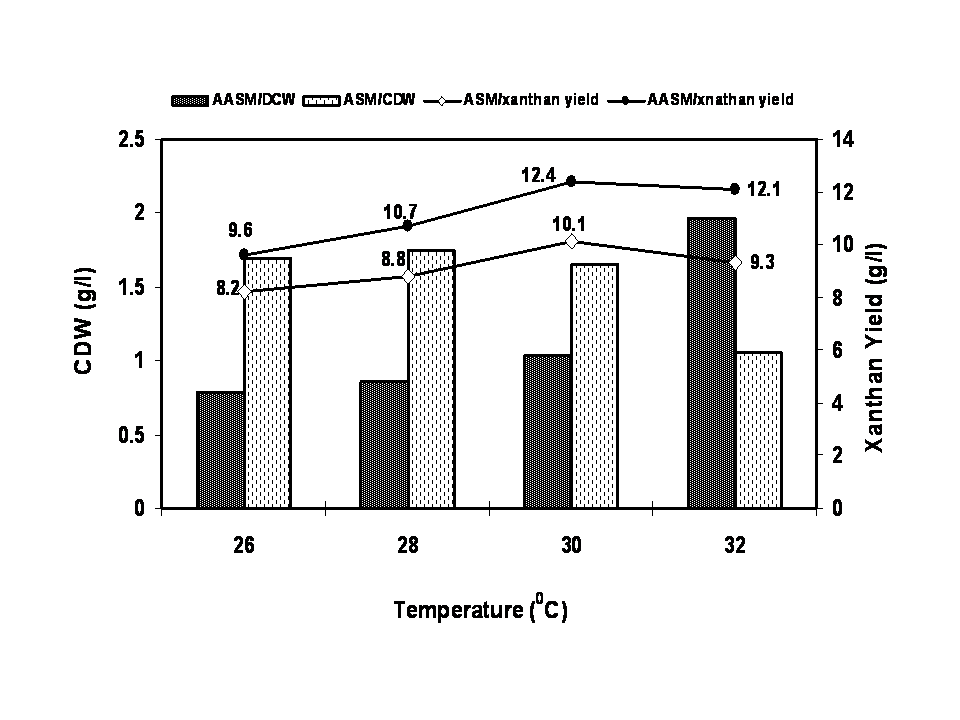 | Figure 3. Optimization of temperature for xanthan production using Xanthomonas campestris |
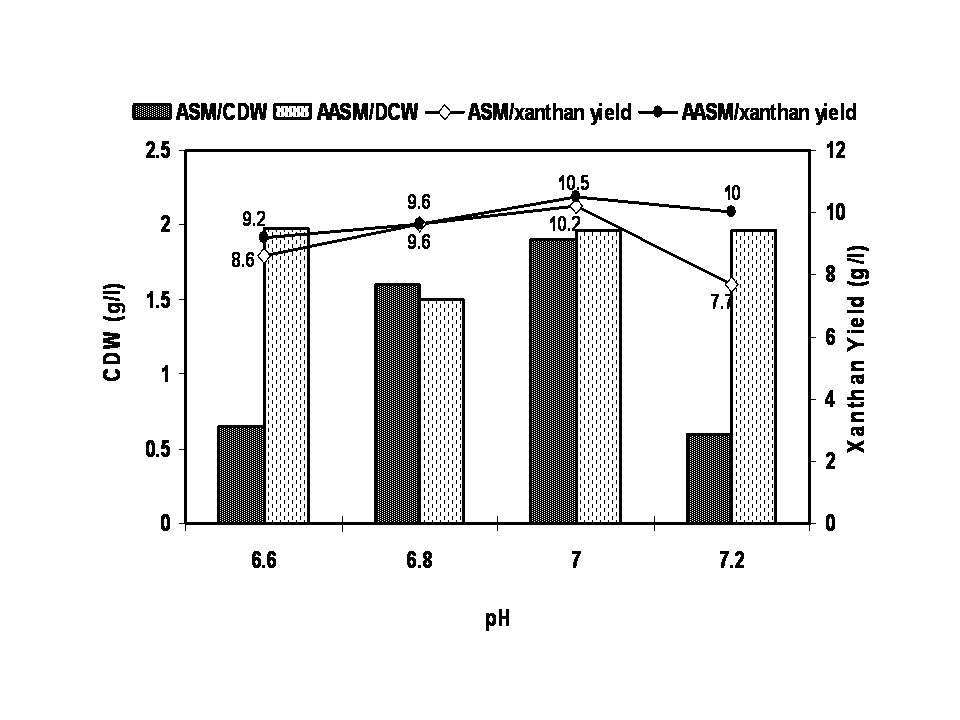 | Figure 4. Optimization of pH for xanthan production using Xanthomonas campestris |
4. Conclusions
- To meet the demand of xanthan requirement due to many industrial applications, low cost medium is required for the fermentation. The xanthan production was increased successfully after optimization studies, using different concentrations of ASM, AASM, yeast extract with initial pH, temperature (37℃) and incubation period (48 h). Maximum yield was achieved even in 1% AASM with 1% yeast extract as nitrogen source at 30℃. X. campestris produced xanthan yield of 12.3 g/l using AASM. This study concluded that the growth parameters and carbon sources chosen increased the production of xanthan by X. campestris.
ACKNOWLEDGEMENTS
- We greatfully acknowledge the faculty of the School of Chemical and Biotechnology, Sastra University, Thanjavur for the technical help.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML