-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
2012; 2(2): 19-30
doi: 10.5923/j.ajee.20120202.04
Evaluation of Spatial and Seasonal Variations in Groundwater Quality at Kolar Gold Fields, India
Piyush Gupta , Surendra Roy
National Institute of Rock Mechanics, Kolar Gold Fields, India
Correspondence to: Piyush Gupta , National Institute of Rock Mechanics, Kolar Gold Fields, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Underground gold mining at Kolar Gold Fields (KGF), Karnataka, was carried out for more than a century, which disturbed the groundwater aquifers. In addition, leaching from mill tailings dumps has also been contaminating groundwater in this area. The aim of the study was to assess the groundwater quality and its suitability for drinking purpose. A detailed survey was carried out within a 10 km radius of KGF and 12 borewells, being used for domestic purposes, were identified for groundwater sampling. The samples were collected from each borewell in each season and analysed for different water quality parameters. It was found that pH, total suspended solids, nitrate, arsenic, fluoride, cyanide, sulphate, alkalinity and sodium in groundwater were within the regulatory limits whereas other parameters exceeded the norms. Water quality index (WQI) was evaluated to know the overall quality of groundwater for each borewell for different seasons. Multiple regression model was developed for prediction of WQI and the performance of the model was assessed. Methods for the treatment of groundwater have also been suggested in this paper.
Keywords: Gold mill tailings, Groundwater quality, Kolar Gold Fields, Physicochemical parameters, Seasonal variation, Water Quality Index
Article Outline
1. Introduction
- The Kolar Gold Fields (KGF) mines are seated towards the southern end of a narrow strip of schist in the Kolar district of Karnataka. At KGF, gold has been mined to a depth of 3 km below the surface with 650 km of tunnel work[1] and mining was carried out over 120 years and about 40 million tonnes of mill tailings were accumulated[2].At KGF, groundwater is a major source of water supply for domestic purposes. Huge amounts of mill tailings dumps, sewerage system in township areas, agriculture and earlier mining activities can contaminate groundwater quality. The use of contaminated groundwater for drinking purpose can cause health problems.The water quality index (WQI) has been widely used to characterize the usability of water resources for domestic purposes. It provides a single number that expresses overall water quality at a certain location and time, based on several water quality parameters. It gives general idea of the possible problem with water in a particular region[3] and is one of the most effective ways to know water quality[4].In this study, borewells frequently being used by the public were surveyed to analyze physical, chemical and biological characteristics of water for the assessment of safe drinking water source, to study seasonal variations in water quality parameters with respect to locations, to evaluate seasonal WQI for different borewells, to develop model for WQI for computation of water quality for any season, and to suggest treatment methods.
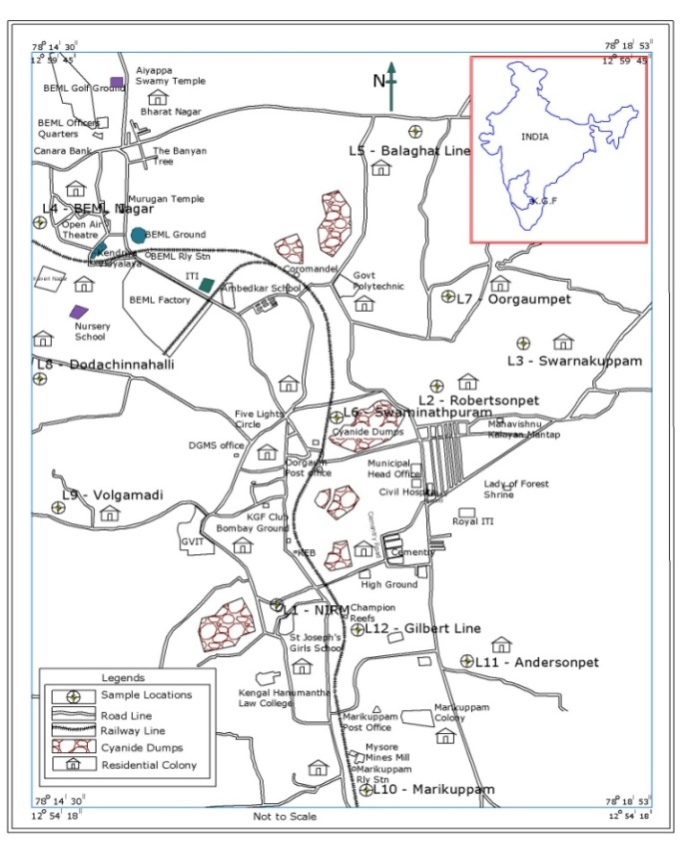 | Figure 1. Sampling locations and other surface structures at KGF |
2. Methodology
2.1. Sampling Locations
- A detailed survey was carried out within a 10 km radius of KGF to identify borewells being used for domestic purposes. Accordingly 12 borewells at different locations (Figure 1) were selected for water sampling, out of which, 5 locations (L4, L5, L8, L9 and L10) are used for drinking in addition to other domestic purposes whereas remaining wells are used for other than drinking purpose. The sampling locations, gold mill tailings dumps, residential colonies and other surface structures are depicted in Figure 1.
2.2. Collection of Samples
- At first, borewells were pumped for 5-10 minutes until the pH remained constant. This phenomenon purged the stagnant water from the aquifers. After purging, fresh aquifer samples were collected[5]. Stoppered polyethylene bottles of two litre capacity were thoroughly cleaned and rinsed with water being sampled. The samples collected by grab sampling techniques from different locations were sealed and labeled properly. The samples were collected in different seasons like post-monsoon (October-November), winter (January-February), summer (April-May) and monsoon (July-August) during 2009-10. Preservations of samples were done as per the standard procedures[6]. Sample from each location was collected two times in each season at 30 days interval to determine the average value of the results, which were compared with drinking water standards.For determination of bacterial contamination, samples were collected in sterile bottles of 200 ml capacity in winter. The samples were kept into ice box and immediately brought to the laboratory for analysis.
2.3. Water Quality Parameters Tested
- The samples were analyzed following the methods given in Standard Methods for the Examination of Water and Wastewater[6]. In situ measurements of temperature, electrical conductivity, dissolved oxygen, pH and turbidity were made with Water and Soil Analysis Kit. Total suspended solids were estimated by filtering a known volume of water. Total dissolved solid was determined using digital TDS meter. Total Kjeldahl nitrogen was analysed by Kjeldahl digestion assembly, ammonical nitrogen by colorimetric method, nitrate by phenol disulfonic acid method, arsenic by water hydride generation atomic absorption spectrometric method, fluoride by SPADNS spectrophotometric method and chloride by argentometric titration method. Sulphate and sulphide were analysed by turbiditmetric and iodometric method respectively. Acidity and alkalinity was determined by titration. Calcium, magnesium and total hardness were measured by Ethylene Diamine Tetra Acetic Acid (EDTA) titration method. Sodium and potassium was determined using flame photometer. Biochemical Oxygen Demand (BOD) was measured by direct method using BOD digital incubator and Chemical Oxygen Demand (COD) by digital reactor and photometer. Cyanide was analysed by colorimetric method and coliform organisms by membrane filtration technique. All the parameters were analysed within one week of sample collection after preservation.
2.4. Water quality index
- WQI is defined as a rating reflecting the composite influence of different water quality parameters on the overall quality of water. It indicates the quality by an index number, which represents the overall quality of water for any intended use[7]. It depends upon weight or weighting and quality rating of different water quality parameters. The purpose of assignment of weights to water quality variables is to denote each variable's importance to the overall water quality. A larger weight value implies greater importance of the variable and depends on the standards in drinking water set by National and International agencies. Those parameters, which have low standards, can influence the water quality to a large extent even a little fluctuation shows high weighing. The parameter having high limits are less harmful to the water quality allocates low weighting[8]. Therefore, the weights for various water quality characteristics are assumed to be inversely proportional to the recommended standards for the corresponding parameters.To determine the overall quality of water at different locations in different seasons, WQI was calculated based on the methods given by Sindhu & Sharma[9], Alobaidy et al.[10] and Sisodia & Moundiotiya[7], which is as under:
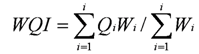 | (1) |
|
3. Results and Discussion
- In the discussion, the water quality parameters have been compared with the drinking water standards prescribed by the Bureau of Indian Standards (BIS)[11]. The parameters for which BIS standards are not available have been compared with United States Public Health (USPH)[12] and World Health Organization[13] standards. The available standards of parameters have been written in their respective figure for comparison and parameters for which standards are not available neither mentioned in the figure nor in the text.
3.1. Assessment of Water Quality Parameters
- pH is a numerical expression that indicates the degree to which water is acidic or alkaline. It causes chemical reactions such as solubility and metal toxicity depending on its concentrations. It did not vary significantly in the seasons i.e. the results were very uniform throughout this study, with minor spatial and seasonal differences (Figure 2a). All values lied within the tolerance limit. The lower pH value tends to make water corrosive and higher pH provides taste complaint and negative impact on skin and eyes[14].The highest temperature (28.25℃) was at location L9 in summer and the lowest (24.9℃) at L1 in monsoon indicating seasons influenced the temperature (Figure 2b). Variations in solar energy received at the earth’s surface create periodicities, both diurnal and annual, in temperature below ground surface[15]. Season and topography influences the temperature[16]. Total suspended solids (TSS) consist of silt, clay and fine particles of organic and inorganic matter that usually remains on the filter paper[17]. Seasonal changes showed the lowest (0.147 mg/l) value at L1 in monsoon and the highest (1.513 mg/l) at L6 in winter (Figure 2c). Though mine is closed but earlier activities might have caused its presence in accumulated water. The observed values are within the permissible limit of USPH[12].Total dissolved solids (TDS) mainly consists of inorganic salts such as carbonates, bicarbonates, chlorides, sulphates, phosphates and nitrates of calcium, magnesium, sodium, potassium, iron etc. and small amount of organic matter[18]. At most of the locations, TDS was higher in winter and lower in post-monsoon (Figure 2d) indicating influence of seasons on concentrations. Wavde & Arjun[19] also found seasonal changes in groundwater. TDS exceeded the tolerance limit at all locations except L9 in post-monsoon. Dissolution of calcite and other minerals might be contributing TDS at different locations[1]. Beyond the limit, palatability decreases and causes gastro intestinal irritation[20], during cooking forms scales in the cooking vessels[21].Electrical conductivity (EC) measures the amount of ions in a solution. It is an indirect measure of the total dissolved solids content of water[14]. Seasonal variations indicated higher conductivity at all locations in winter compared to other seasons (Figure 2e). It exceeded the standard of USPH[12] at all locations indicating high salinity in groundwater. This might be due to the presence of inorganic dissolved solids such as chloride, sulfate, sodium, magnesium, calcium, and iron cations[14]. According to Ullah et. al.[22], movement of salts along with percolation of water causes EC in groundwater. Often groundwater has higher EC compared to surface water. Excess values lead to scaling in boilers, corrosion and quality degradation of the product[23].Turbidity depends on the nature of the water bodies such as river under flood conditions, lake or other water existing under relatively quiescent conditions. Under quiescent conditions, most of the turbidity is due to colloidal and extremely fine dispersions[24]. At most of the locations, turbidity is within the norm except L2 and L10 (Figure 2f). High turbidity shortens the life of sand filters and increases cleaning costs of the filters[24].
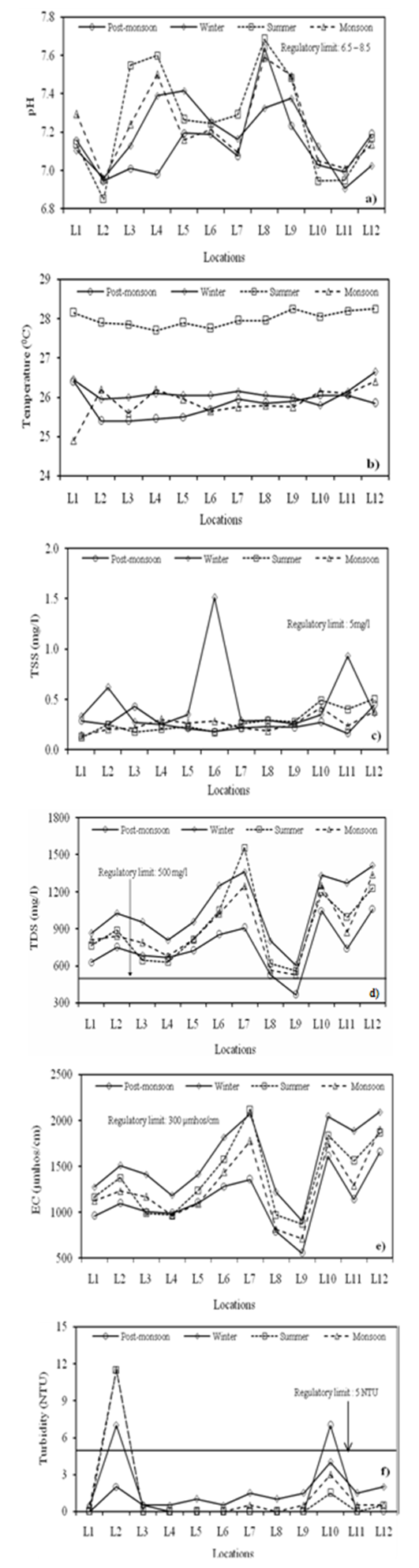 | Figure 2. Seasonal variations in (a) pH (b) Temperature (c) TSS (d) TDS (e) EC and (f) Turbidity at different locations |
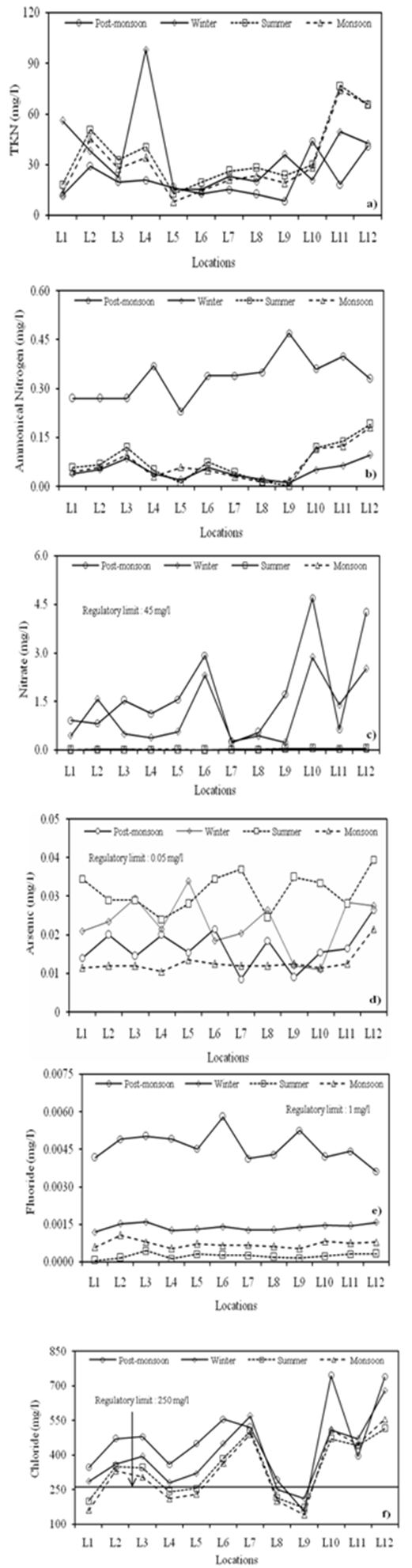 | Figure 3. Seasonal variations in (a) TKN (b) Ammonical nitrogen (c) Nitrate (d) Arsenic (e) Fluoride and (f) Chloride at different locations |
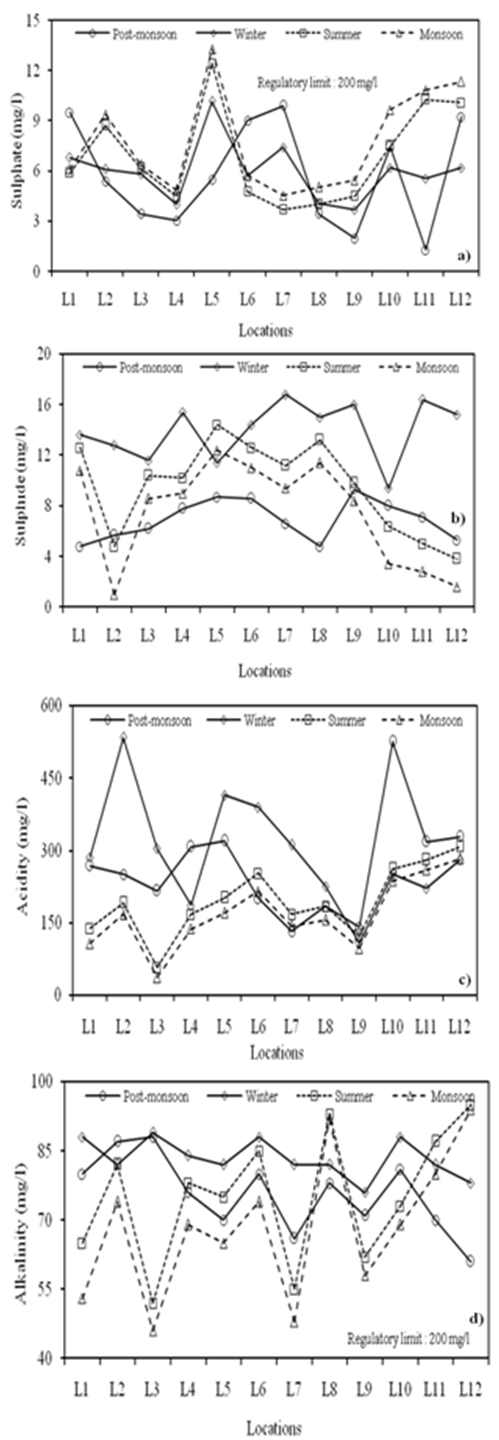 | Figure 4. Seasonal variations in (a) Sulphate (b) Sulphide (c) Acidity and (d) Alkalinity at different locations |
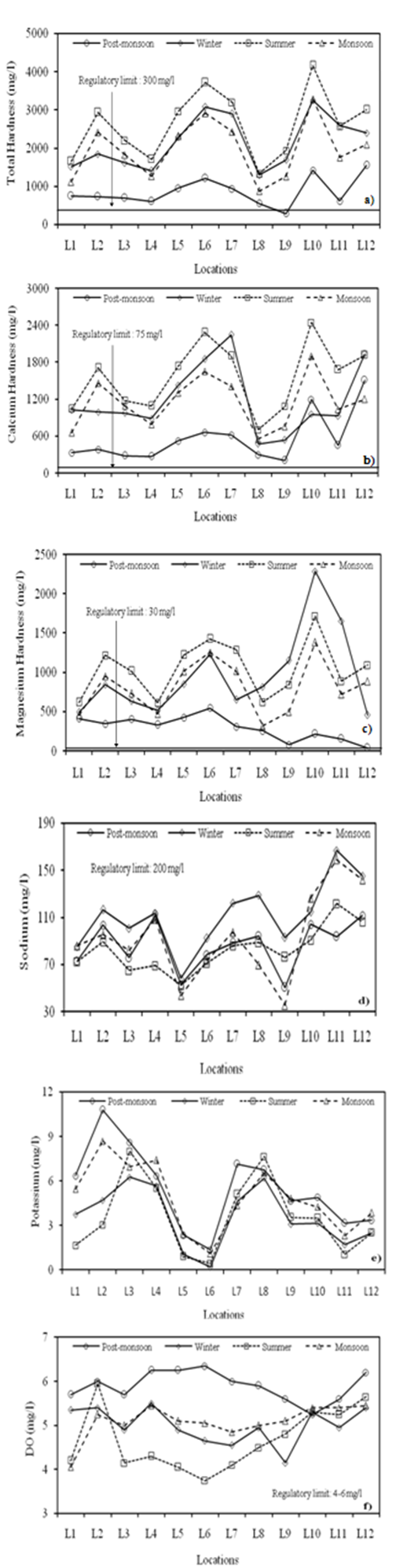 | Figure 5. Seasonal variations in (a) Total hardness (b) Calcium (c) Magnesium (d) Sodium (e) Potassium and (f) DO at different locations |
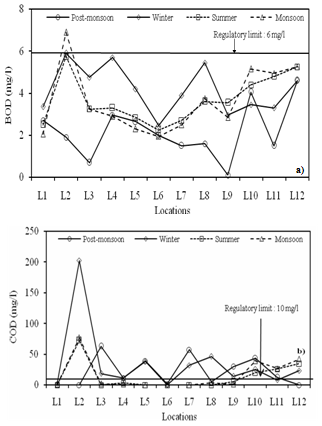 | Figure 6. Seasonal variations in (a) BOD and (b) COD at different locations |
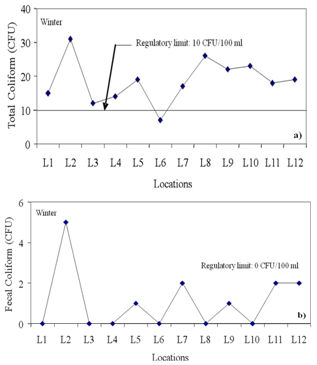 | Figure 7. Variations in (a) total coliform and (b) fecal coliform at different locations |
3.2. Assessment of WQI
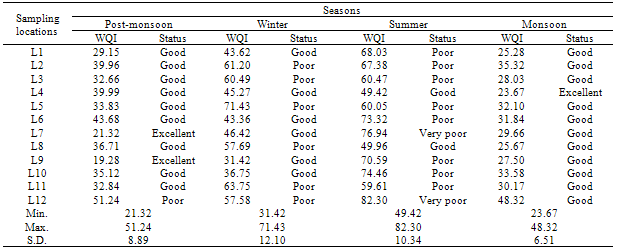
- Many researchers have considered different water quality parameters for the assessment of water quality index [8]. In this study, those parameters for which standards are available like pH, TSS, TDS, EC, turbidity, nitrate, fluoride, arsenic, chloride, sulphate, alkalinity, total hardness, Ca- hardness, Mg-hardness, sodium, DO, BOD and COD were considered for the assessment of WQI in post-monsoon, winter, summer and monsoon. Though total and fecal coliform was analysed in winter season but only total coliform was incorporated in index assessment. Since standard of fecal coliform is zero, it was not considered in index determination.WQI evaluated for 12 sampling locations in different seasons are given in Table 2. The minimum and maximum values indicate the range of water quality at different locations in different seasons. In summer, the quality at most of the locations was poor compared to other seasons. In monsoon, the range occurred under good to excellent category. The dilution properties due to rain might be the reasons for improved water quality in monsoon. It was observed that even at the same monitoring location the quality of water varied from season to season. At location L4, the water quality is excellent in monsoon and good in other seasons. At L7, it is excellent in post-monsoon, good in winter and monsoon but degraded (very poor) in summer. Similarly water quality at L9 was excellent in post-monsoon, good in winter and monsoon but degraded (poor) in summer. Hence, seasons influenced the water quality. Low standard deviation in monsoon shows that fluctuation in water quality index at different locations is lesser than other seasons. In winter, WQI ranged from 30.43 to 70.89 after removing total coliform whereas it varied from 31.42 to 71.43 after incorporating (Table 2) indicating that there are no differences in water quality status.
3.3. Multiple Linear Regression Analysis of Data
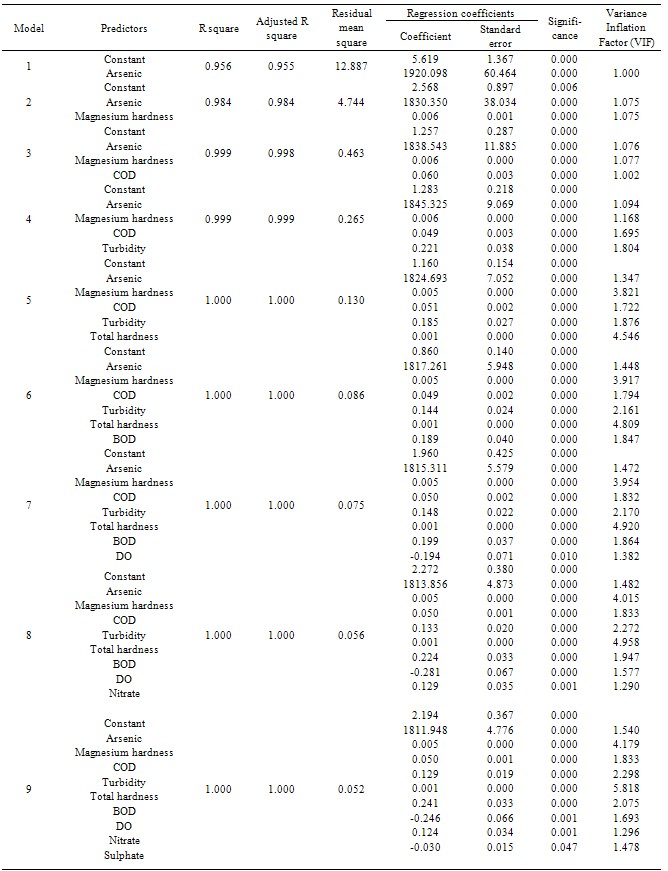
- To determine the most influencing parameters as well as to develop a multiple linear regression model for the assessment of WQI from 48 sets of data, SPSS software version 13.0 was used. Stepwise regression algorithm was followed for the selection of variables. According to Grivas & Chaloulakou[39], stepwise multiple regression procedure is commonly used to produce a parsimonious model that maximizes accuracy with an optionally reduced number of predictor variables.
|
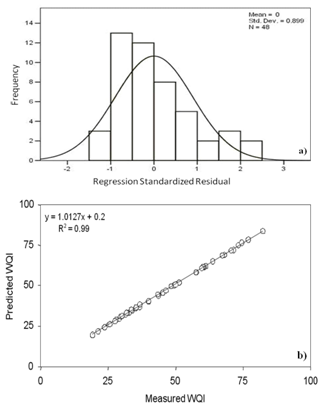 | Figure 9(a). Standardized residual analysis and (b) Correlations between predicted and measured values of WQI |
|
3.4. Water Treatment Methods
- At KGF, groundwater is the only source of water supply for domestic purpose but it cannot be used for drinking purpose without treatment. Analysis showed that some parameters like TDS, hardness, electrical conductivity, etc. exceeded the limits. The methods developed by different researchers can be use for the treatment of water. The temporary hardness caused by the carbonates and bicarbonates of calcium and magnesium can be removed by boiling or by adding lime. The permanent hardness is caused by the sulphates, chlorides and nitrates of calcium and magnesium, which can be removed by special methods of water softening like lime-soda, Zeolite, demineralization[41], electrodialysis[42,43] etc. Dissolved salts in water can be removed by methods like electrodialysis, reverse osmosis, freezing process, etc[41]. TDS in groundwater was observed below 2000 mg/l indicating that electrodialysis method can be used for treatment[41]. Electrodialysis is a compact machine, cost of buying and erecting the plant is small, easy to operate and can be started and stopped in a few minutes. This is an ideal machine for purifying water in a small town or at a remote place.
4. Conclusions
- The analysis of water from 12 different borewell locations of KGF showed that TDS, EC, hardness, coliform bacteria, etc. exceeded the norms at most of the locations. Presence of sewer lines and septic tanks adjacent to borewells caused higher BOD and COD at some locations. Cyanide is nil in water samples.Water quality index showed good water quality in monsoon and poor in summer because most of the parameters were within the norms in monsoon compared to other seasons. Its evaluation will not only be helpful to understand the seasonal quality of water but also has advantages for government agencies and institutions where regular water quality data is required. The model developed for WQI can be used to evaluate water quality for any season of the study area. Total hardness, magnesium hardness, DO, BOD, COD, turbidity, arsenic, nitrate and sulphate are the most influencing parameters. Hence testing of only these parameters will be sufficient for the evaluation of WQI.Based on the results and analysis of water samples, it is recommended to use water only after boiling and filtering or by Reverse Osmosis treatment for drinking purpose by the individuals. For the treatment of water on large scale basis, it is suggested to install electrodialysis treatment plant at KGF. However, for other domestic purposes, water of different borewells can be used if the WQI shows status of water quality either good or excellent.
ACKNOWLEDGEMENTS
- The authors are thankful to the Director, National Institute of Rock Mechanics (NIRM) for his technical guidance and in-house funding of this project. They are also grateful to Dr. G. R. Adhikari, Scientist & Head, Technical Coordination & Project Management Department, NIRM for his valuable comments.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML