-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Environmental Engineering
2012; 2(2): 1-5
doi: 10.5923/j.ajee.20120202.01
Treatment of Municipal Landfill Leachate by Oxidants
Shabiimam M. A. 1, Anil Kumar Dikshit 1, 2, 3
1Centre for Environmental Science and Engineering, IIT Bombay, Mumbai, 400076, India
2School of Civil Engineering, Survey and Construction, University of KwaZulu-Natal, Durban, 4041, South Africa
3School of Civil and Environmental Engineering, Nanyang Technological University, 639798, Singapore
Correspondence to: Anil Kumar Dikshit , Centre for Environmental Science and Engineering, IIT Bombay, Mumbai, 400076, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Landfilling is the one of the least expensive method for disposal of municipal solid waste. Hence, about 90% of MSW is disposed in open dumps and landfills unscientifically, creating problems to public health and the environment. The leachate generated from the municipal landfill contains organic and inorganic pollutants and several heavy metals, which makes it unsuitable for discharge in natural bodies without any prior treatment. In this study, matured landfill leachate was treated by various oxidation and combination of advanced oxidation process using hydrogen peroxide, sodium persulfate, Fenton’s oxidation and ultrasonication-hydrogen peroxide. Fenton’s process exhibited the best COD, TOC and color removals(74% COD, 76% TOC and 80% color respectively) while hydrogen peroxide and sodium persulfate alone showed much lesser TOC reduction(35% and 39% respectively).
Keywords: Landfill Leachate, Fenton Process, Advanced Oxidation Process, Sodium Persulfate, Ultrasonication
Article Outline
1. Introduction
- Municipal solid waste (MSW) is generated from the waste materials discarded from domestic, commercial, institutional, market and other sources. Food waste, paper, plastic, newspaper, rubber, leather, tin cans, scrap ferrous and nonferrous metals are normal component of MSW. However it might contain hazardous substances like paints, mercury, pharmaceuticals, batteries, and many other items. Municipal landfill is considered to be most common and least expensive method of disposing urban wastes. It is reported that about 90% of MSW is disposed in open dumps and landfills unscientifically, creating problems to public health and the environment[1]. If landfills are not properly managed, these can give uncontrolled gaseous and liquid emissions. Liquid emission is termed as ‘leachate’ and it may contain several organic and inorganic compounds and heavy metals. It has been observed that the composition of leachate is dependent on the age of the landfill and the characteristics of disposed waste[2]. The proper treatment and safe disposal of the leachate is one of the major environmental challenges worldwide especially in developing countries like India.
1.1. Chemical Oxidation
- Chemical oxidation method is used for treatment of leachate containing refractory compounds. In many cases, oxidation was used for the treatment of matured leachate, still having high amount of recalcitrant chemicals[3]. In past few decades, Advanced oxidation process(AOP) is used for the wastewater treatment. It helps to remove the color and organic load from leachate[4,5]. The main attraction is that most of the advanced oxidation processes do not generate sludge[6]. Common AOP processes are based on oxidizing agents like O3, H2O2 and combination of O3 and H2O2. Irradiation techniques are also used to treat the effluent using ultrasonication, microwave, ultraviolet or combination of oxidizing agent with O3. Rivas et al.(2003) investigated the treatment of leachate by ozonation. In 1 h duration, 30% COD removal was observed whereas after 120 h, 90% of COD removal was noticed[7]. The oxidation potential of hydroxyl radicals(2.8 V) is higher than that of ozone(2.07 V). In addition, Fe2+ and Fe3+ are also known coagulants, which give this process dual advantage of oxidation and coagulation. Yokoyama et al.(2009)[6] investigated the leachate treatment by AOP at initial COD of 5400 mg/L and Fenton oxidizing agent of Fe2+(at 720 mg/L) was used. 80% of COD removal was achieved at pH 4 from this process[6]. Other significant advantages of this process over other advanced oxidation processes are non-toxic nature and abundant availability of iron compounds and ease to handle hydrogen peroxide[8]. Lopez et al. (2004) investigated municipal landfill leachate treatment using Fenton process. At optimum H2O2 dose of 10000 mg/L and Fe2+ dose of 830 mg/L, 60% COD removal was acheived[9].Table 1 shows the performance of Fenton process for treatment of leachate reported in literature.
2. Materials and Methods
2.1. Wastewater
- Landfill leachate was collected from Municipal dumping yard at Deonar in Mumbai, India and was brought to Environmental Laboratory, IIT Bombay, India. The leachate was stored at 4℃ in cold room till experimentation. The characterisation of leachate was done as per standard methods[14].
2.2. Oxidants
- Common oxidizing agents viz. hydrogen peroxide, sodium persulfate, and Fenton’s reagent were tested for their suitability for COD, TOC and color removal from the leachate. Hydrogen peroxide solution (30%) and ferrous sufate were procured from Merck India limited, Mumbai; while sodium persulfate was provided by Moly Chem Limited, Mumbai.
2.3 Analytical Methods
- COD was measured by closed reflux method using HACH COD Digester (DRB 200, USA) as given in Standard Methods[14]. Samples were suitably diluted with ultra pure water. Shuimadzu Total Organic Carbon Analyzer (TOC- VCSH, Japan) was used for TOC determination from 4 ppb to 25000 ppm. pH of the leachate was measured using digital Thermo Scientific pH Meter(Orion 3 Star, Singapore). Intensity of color was measured in terms of absorbance at 356 nm, which is the characteristic wavelength of brown color. Since apparent color is a significant function of pH, samples were diluted in 1 M phosphate buffer to maintain pH of 7. Samples were centrifuged at 10000 rpm for 10 minutes prior to absorbance determination for eliminating hindrance due to suspended particulates in the sample. After that, the supernatant was diluted 10 times and the absorbance was measured at 356 nm using Thermo Spectronic Visible Spectrophotometer (Helios Epsilon, USA). Reduction in color was estimated in terms of reduction in absorbance with reference to that of the control (original leachate). Heavy metals present in untreated and treated leachate were determined with the help of Jobin Yvon Horiba Inductively Coupled Plasma Spectrophotometer (ULTIMA 2000, France)
2.4. Hydrogen Peroxide
- The batch studies were conducted on 200 mL of leachate samples in 500 mL beakers using jar test apparatus at 250 rpm for 1 h. pH was varied from 2 to 4.5 by adding HCl to find optimum pH. At optimum pH, dose of hydrogen peroxide was varied from 9 to 21 mL/L to determine optimum dose. Samples were drawn after 20 minutes and assessed for TOC and color.
2.7. Sodium Persulfate
- Now, sodium persulfate was used as oxidant and experiments were done same as above. Here dose used varied from 9 to 24 g/L.
2.5. Fenton’s Oxidation
- For assessing efficacy of Fenton’s oxidation towards COD reduction, first optimum pH was found by performing jar test at 250 rpm with constant dose of H2O2 (10 mL/L) and FeSO4 (3 g/L) and varying pH from 2 to 4.5. Taking pH constant at optimum and dose of FeSO4 same as above, second run of jar test was done from 6 to 21 mL/L to optimize the dose of H2O2. In the third run, the dose of FeSO4 was varied from 1 to 6 g/L to optimise it taking optimum dose of H2O2 at optimum pH. A pinch of manganese dioxide was added to samples for remove effect of residual H2O2 before measuring its COD. TOC and colour were also monitored.
2.6. Ultrasonication-Hydrogen Peroxide
- Studies were also carried out to find the effect of combined treatment with ultrasound and hydrogen peroxide. 200 mL of leachate sample was treated using 20 Hz frequency ultrasonicator with various concentration of hydrogen peroxide to find the optimum pH and dose.
3. Result and Discussion
3.1. Characteristics of Landfill Leachate
- Initial pH was of landfill leachate was 7.9, turbidity was 317 NTU while COD was 2451 mg/L and BOD was 202 mg/L and the BOD/COD was 0.09. From the pH and BOD/COD ratio, it can be concluded that the leachate had already achieved matured phase and it was not suitable for biological treatment[15]. Table 1 shows the amount of heavy metals present in the leachate. In mature phase, most of the heavy metals concentrations were low because of the dissolution[12].
|
3.2. Hydrogen Peroxide
- Hydrogen peroxide, most popular oxidising agent being used in various industrial applications, was tried as oxidant for leachate.
3.2.1. Effect of pH on the Oxidation of Leachate using Hydrogen Peroxide
- At first step, 12 mL/L hydrogen peroxide was mixed per 200 mL of landfill leachate and its pH was adjusted from 2 to 4.5 with the help of HCl. Figure 1a shows the variation in the TOC and color removal of the sample with respect to its pH. Optimum pH was found as 2.
3.2.2. Effect of Hydrogen Peroxide Dose on the Oxidation of Leachate
- At optimal pH, varying doses of hydrogen peroxide were added to landfill leachate and the COD and color removal efficiency were determined. Color removal increased with increase in the dose of hydrogen peroxide but at higher doses beyond 15 mL/L, there was little increase in the color removal (Figure 1b). In jars having high doses, there was so excessive foaming that they could be operated with great difficulty. Keeping operational ease and economics of treatment in mind, the dose of 18 mL/L was suggested which gave a 39% reduction in TOC and 34% reduction in color.
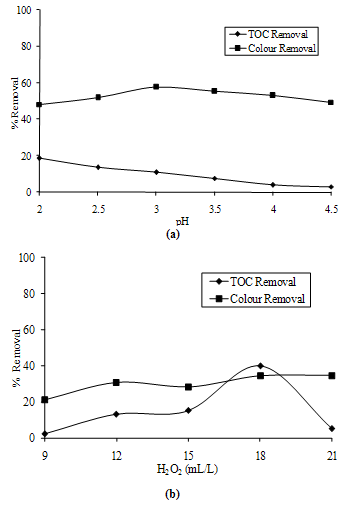 | Figure 1. TOC and Color Removals by Oxidation of H2O2(a) at Various pH and(b) at Various Doses of Hydrogen Peroxide |
3.3. Sodium Persulfate
- Sodium Persulfate is another highly reactive oxidant. It was also tried as oxidant for treatment of landfill leachate.
3.3.1. Effect of pH on the Oxidation of Leachate using Sodium Persulfate
- At first step, 12 g/L of Sodium Persulfate was mixed per 200 mL of landfill leachate and its pH was adjusted from 2 to 4.5 with the help of HCl. Figure 2a shows the variation in the color and TOC removal of the treated sample with respect to its pH. Optimum pH was found as 2.
3.3.2. Effect of Sodium Persulfate Dose on the Oxidation of Leachate
- At optimal pH, varying doses of sodium persulfate were added to landfill leachate and the TOC and color removal efficiency were determined. Color removal increased with increase in the dose of hydrogen peroxide but at higher doses beyond 18 g/L, there was little decrease in the color removal (Figure 2b). The dose of 18 g/L was suggested which gave an optimum removal of 35% in TOC and 79% in color.
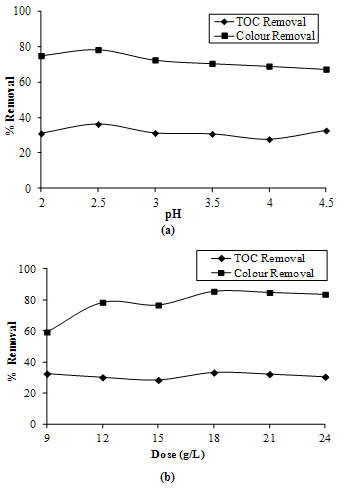 | Figure 2. TOC and Color Removals by Oxidation using Sodium Persulfate(a) at Various pH(b) at Various Doses of Sodium Persulfate |
3.4. Fenton’s Oxidation
- Fenton’s oxidation is very promising option for oxidation of toxic and refractory organic matter. Fenton’s reaction at acidic pH produces ferric ions and hydroxyl radicals. The formation of hydroxyl radical and oxidation of Fe2+ to Fe3+ state is responsible for its high oxidation potential.
3.4.1. Effect of pH on the Fenton’s Oxidation of Leachate
- Many of the literature revealed Fenton’s oxidation process takes place in the acidic pH range, and hence the test for optimum pH was considered in this range. In six jars with winter leachate samples, pH was adjusted from 2 to 4.5 with increment of 0.5 with the help of HCl. After that, constant dose of 10 mL/L hydrogen peroxide and 3 g ferrous sulfate was added per litre of leachate. Jars were subjected to standard test as described in methodology for Fenton’s oxidation process. COD, TOC and color removals were noted and based on that, the optimum pH of 4.5 was selected (Figure 3a).
3.4.2. Effect of Hydrogen Peroxide Dose on Fenton’s Oxidation of Leachate
- At optimum pH of 4.5, the exercise was again repeated to determine optimum dose of hydrogen peroxide. In this step, pH was kept constant as 4.5 along with dose of ferrous sulfate (3 g/L). Dose of hydrogen peroxide was varied from 6 to 21 mL/L.Figure 3b shows the pattern of COD, TOC and color removal. Optimum dose of hydrogen peroxide was found as 12 mL/L. At higher doses, there was no further increase in color removal.
3.4.3. Effect of Ferrous Sulphate Dose on Fenton’s Oxidation of Leachate
- In the study, the pH as well as dose of hydrogen peroxide were kept as constant (i.e. 4.5 and 12 mL/L) and the dose of ferrous sulfate was varied. As is clear from the results shown in Figure 3c, the best combination for Fenton’s oxidation was found as pH 4.5, 12 mL/L H2O2 and 3 g/L ferrous sulphate, which gave maximum reduction of 74% in COD, 76% in TOC and 80% in color of leachate.
3.5. Ultrasonication-H2O2
- Ultrasonication and Hydrogen peroxide, another combination of advanced oxidation used in various industrial applications, was also tried as oxidant for leachate.
3.5.1. Effect of pH on Ultrasonication of Landfill Leachate
- At first step, the beaker contains 200 ml of sample was kept in a sonicator for 1 h, 12 mL/L Hydrogen peroxide was mixed per 200 mL of landfill leachate and its pH was adjusted from 2 to 4.5 with the help of HCl. Figure 4a shows the variation in the organic pollutant removal of the sample with respect to its pH. Optimum pH was found as 2.
3.5.2. Effect of Hydrogen Peroxide Dose on Ultrasonication of Landfill Leachate
- At optimal pH, varying doses of hydrogen peroxide were added to landfill leachate and the TOC and color removal efficiency were determined. Color removal decreased with increase in the dose of hydrogen peroxide but at dose of 12 ml/L maximum TOC and color removal was achieved (Figure 4b). A high dose of H2O2 has given excessive foaming that they could be operated with great difficulty. Keeping operational ease and economics of treatment in mind, the dose of 12 mL/L was finalized with a 36% reduction in TOC and 79% reduction in color.
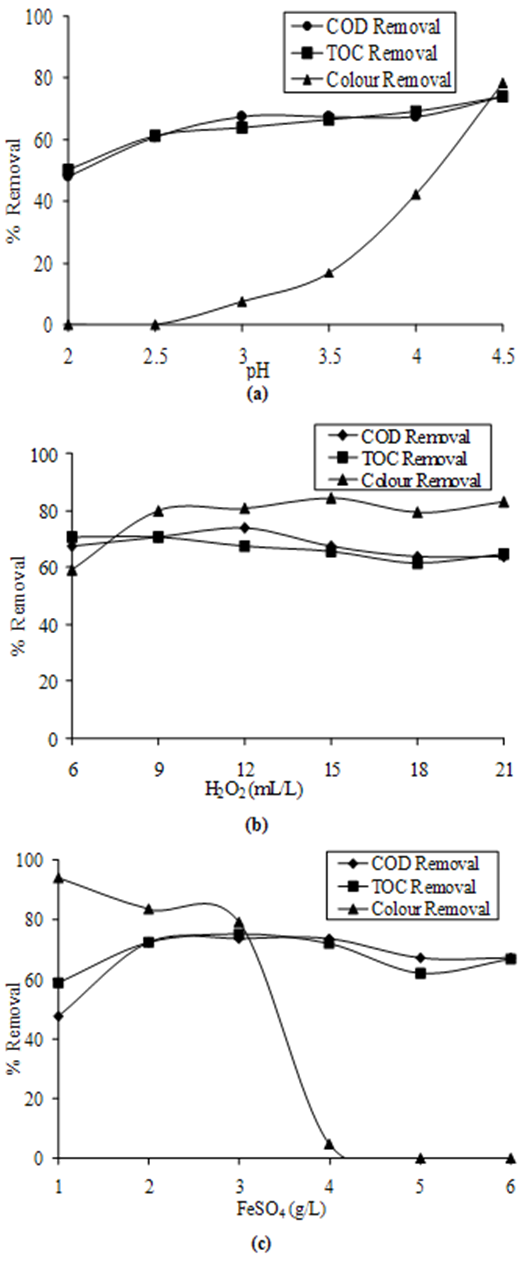 | Figure 3. COD, TOC and Color Removals by Fenton’s Oxidation(a) at Various pH(b) at Various Doses of H2O2(c) at Various Doses of FeSO4. |
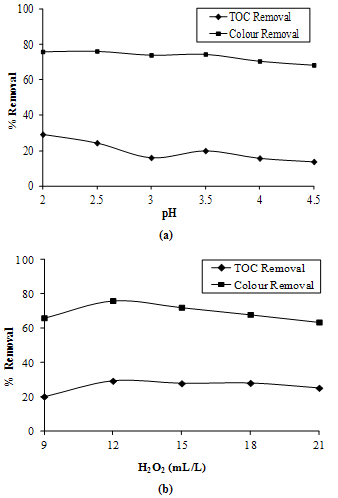 | Figure 4. TOC and Color Removals by Ultrasonication Combined with Hydrogen Peroxide(a) at Various pH(b) at Various Doses of H2O2 |
5. Conclusions
- Hydrogen peroxide alone could hardly remove 39% TOC and 34% color at optimum pH of 2 with optimum dose as 18 mL/L. Combination of ultrasonic destruction and hydrogen peroxide was also found to be not effective, it could remove 36% TOC and 79% color at optimum pH of 2 and optimum dose of 12 ml/L. TOC and color removal by oxidation of sodium persulfate also did not achieved better removal (35% TOC and 79% color). From the experimental results, the oxidant alone could remove the TOC in the range of 35-39%.Fenton’s oxidation gave the best results with total removal of 74% COD, 76% TOC and 80% color at optimum initial pH of 4.5 with optimum doses 12 mL/L hydrogen peroxide and 3 g/L of ferrous sulfate.
ACKNOWLEDGEMENTS
- The authors thank the management and staff of the Solid Waste Division, Municipal Corporation of Greater Mumbai, Mumbai, India for providing the landfill leachate used in the research work.
References
| [1] | Sharholy, M., Ahmad, K., Mahmood, G., Trivedi, R. C., 2008, Municipal solid waste management in Indian cities - A review., Waste Management, 289(2), 459-67 |
| [2] | Baig, S., Coulomb, I., Courant, P., Liechti, P., 1999, Treatment of Landfill Leachates: Lapeyrouse and Satrod Case Studies, Ozone: Science & Engineering, 21(1), 1-22 |
| [3] | Cortez, S., Teixeira, P., Oliveira, R., and Mota, M., 2011, Evaluation of Fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments., Journal of Environmental Management, 92(3), 749-55 |
| [4] | Wu, Y., Zhou, S., Qin, F., Peng, H., Lai, Y., Lin, Y., 2010, Removal of humic substances from landfill leachate by Fenton oxidation and coagulation, Process Safety and Environmental Protection, 88(4), 276-284 |
| [5] | Renou, S., Givaudan, J. G., Poulain, S., Dirassouyan, F., Moulin, P., 2008, Landfill leachate treatment: Review and opportunity., Journal of Hazardous Materials, 150(3), 468-93 |
| [6] | Yokoyama, L., Araujo, F. V. F., Campos, J. C., Freire, L. F. A., Teixiera, I. A. C., 2009, Landfill Leachate Treatment with Advanced Oxidation Process, Proceedings Sardinia, Twelfth International Waste Management and Landfill Symposium, S. Margherita di Pula, Cagliari, Italy |
| [7] | Rivas, F. J., Beltrán, F., Carvalho, F., Acedo, B., Gimeno, O., 2004, Stabilized leachates: sequential coagulation- flocculation + chemical oxidation process., Journal of Hazardous Materials, 116(1-2), 95-102 |
| [8] | Benatti, C. T., Tavares, C. R. G., Guedes, T. A., 2006, Optimization of Fenton’s oxidation of chemical laboratory wastewaters using the response surface methodology, Journal of Environmental Management, 80(1), 66-74 |
| [9] | Lopez, A., Pagano, M., Volpe, A., Di Pinto, A. C., 2004, Fenton’s pre-treatment of mature landfill leachate, Chemosphere, 54, 1005–1010 |
| [10] | Wang, P., Lau, I., Fang, H., Zhou, D., 2000, Landfill leachate treatment with combined uasb and fenton coagulation. Journal of Environmental Science and Health, Part A, 35(10), 1981-1988 |
| [11] | Barnes, D., Li, X., Chen, J., 2007, Determination of Suitable Determination of suitable pre-treatment method for old-intermediate landfill, Environmental Technology, 28(2), 195-203 |
| [12] | Yilmaz, T., Aygün, A., Berktay, A., Nas, B., 2010, Removal of COD and color from young municipal landfill leachate by Fenton process, Environmental Technology, 31(14), 1635–1640 |
| [13] | Barbusi, K., and Pieczykolan, B., 2010, COD removal from landfill leachate using Fenton oxidation and coagulation, Architecture Civil Engineering Environment, 3, 93-100 |
| [14] | “Standard Methods for the Examination of Water and Wastewater, 2005, 2nd ed., APHA, Washington, DC |
| [15] | Kjeldsen, P., Barlaz, M. A., Rooker, A. P., Baun, A., Ledin, A., Christensen, T. H., 2002, Present and long-term composition of MSW landfill leachate: a review, Critical Reviews in Environmental Science and Technology, 32(4), 297–336 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML