-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2022; 11(2): 21-26
doi:10.5923/j.ajdv.20221102.01
Received: Oct. 13, 2022; Accepted: Oct. 31, 2022; Published: Nov. 14, 2022

Role of Biomarker and Personalized Medicine in Atopic Dermatitis Management
Helmina Robiyatul Umami
Faculty of Medicine Muhammadiyah Surakarta University, Surakarta, Indonesia
Correspondence to: Helmina Robiyatul Umami, Faculty of Medicine Muhammadiyah Surakarta University, Surakarta, Indonesia.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Atopic dermatitis (AD) is closely related to the immunological disorder. One of the main therapeutic strategies for atopic dermatitis is to control the immune system. Many biomarkers were identified to have some role in atopic dermatitis progression. Disease biomarkers are important and helpful in the treatment of atopic dermatitis. Biomarkers may identify the best-personalized treatment option for atopic dermatitis management which is known as personalized medicine. Here, we discuss some biomarkers that may relate to a treatment option for atopic dermatitis in different conditions and different patients. Biomarker profile differences in some people may correlate to a different treatment option. Here we discuss the best treatment option for atopic dermatitis based on biomarkers. This study design as a review and was conducted following PRISMA guidelines. Publications were searched in PubMed. 15 studies related to AD biomarkers were found that met the inclusion criteria. Th1 (CXCL9), Th2 (IL-13, CCL17/CCL22), Th17/Th22, IgE and epidermal thickness change significantly in clinical trial treatments. Biomarkers play role as severity assessment and personalized medicine.
Keywords: Atopic dermatitis, Biomarker, Personalized medicine, Management
Cite this paper: Helmina Robiyatul Umami, Role of Biomarker and Personalized Medicine in Atopic Dermatitis Management, American Journal of Dermatology and Venereology, Vol. 11 No. 2, 2022, pp. 21-26. doi: 10.5923/j.ajdv.20221102.01.
1. Introduction
- The prevalence of AD is estimated to be 15-20% in children and 1-3% in adults, and the incidence has increased by 2 to 3 fold during the past decades in industrialized countries [1]. Atopic dermatitis (AD) is caused by a complex interaction of immune dysregulation, epidermal gene mutations, and environmental factors that disrupts the epidermis causing intensely pruritic skin lesions. Repeated scratching triggers a self-perpetuating itch-scratch cycle, which can have a significant impact on the patient's quality of life [2]. In a review of cohort studies, 38% of adults with AD had symptom onset in childhood. Many describe pain, stinging and embarrasing from their AD impacting their choice of clothing. The burden increases with increasing severity of disease [3]. The diagnose tool that use widely worldwide is Hanif and Rajka’s criteria that based on clinical appearance. The severity of AD also identified by tools based clinical appearance, such as SCORing of Atopic Dermatitis (SCORAD), Eczema Area and Severity Index (EASI) and Investigator’s Global Assessment (IGA) that’s rely on the observer’s subjective assessment [4] But it is also known that in the AD patient's skin lesions there is found infiltration of T-helper type 2 (Th2) cells and increased dendritic cells. Dendritic cells induce cytotoxins that activate eosinophils, and Natural Killer (NK) cells [5]. In addition, Th2 cells produce various pro-inflammatory cytokines, including Interleukin (IL)-4, IL-5, and IL-13. IL-4 induced the production of immunoglobulin (Ig) E to develop hypersensitivity reaction [6]. It is clear that the pathogenesis of AD is not only associated with Th2 but also Th1 and Th17. The heterogeneity of Th22 cytokine it is unlikely that novel molecular therapies targeting specific immunologic pathways will be equally effective in all patients with AD, which makes stratification of subtypes of patients with AD of increasingly important [7]. The specific pathway of immunological mechanism in AD lead many study to found specific biomarker. Some biomarkers may relate to a treatment option for atopic dermatitis in different conditions and different patients.
2. Method
- This study design as a review and was conducted following PRISMA guidelines. Publications were searched in PubMed. The following inclusion criteria of the articles were: (a) Articles published in 2017 until 22 September 2022; (b) Keyword used “atopic dermatitis biomarker”; (c) Publication defined as Clinical trials; (d) Publication defined as a Free-full text article; (e) Publication correlate with diagnosis and management of atopic dermatitis disease; (f) English language. Additionally, we conducted a manual search in the references to search the meaning or terms that used in publication.
3. Biomarkers to Diagnostic in DA
- Biomarker comes from the word "biological marker" which means a broad subcategory of medical signs that can be measured objectively. Biomarker difference from clinical signs and could play a significant role in the diagnosis, prognosis and management of AD. WHO defined biomarker as “any substance, structure or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease. Biomarkers; can be classified into markers of exposure, effect, and susceptibility” [4]. The biologic origin of a biomarker could be genomic information, transcriptomic profiles obtained by analysis of mRNA and miRNA, proteins such as cytokines and other mediators from body fluids (whole blood, serum, plasma, tissue fluids) or tape stripping, and morphological information [8].The elevated of serum IL-19 level is known relevance in AD and psoriasis severity [9]. In a cohort study involving Asians, African-American and Caucasians, serum IgE levels were elevated in All Asian patients (>480ng/ml) while among Caucasian and African-American patients the relative distribution for elevated IgE levels was 80%. Asian patients were predominately associated with the low inflammatory subgroup, whereas African-American associated with the high inflammatory subgroup, and Caucasian distributed evenly. There is association to baseline EASI Score, SCORAD Index, Itch Numeric Rating Scale (NRS) and Dermatology Life Quality Index (DLQI). Specifically, the high inflammatory subgroup was associated with higher disease score as compared to the low inflammatory subgroup. The study also found that Th2/Th22-related biomarkers, including IL-13, IL-22, Thymus and activation-regulated chemokine (TARC)/CCL17, Macrophage-derived chemokine (MDC)/CCL22, Monocyte chemo-attractant protein (MCP)-4/CCL13 and MCP-3/CCL17 correlate with disease severity. Furthermore IL-13, MCP-3 and TNFβ were found to be variable in both high inflammatory and low inflammatory subgroup and can be potential biomarker to detect severity of AD [10]. With the skin tape strip (STS) method, IL4R, CCL22, CCR4 also found correlate with severity of AD [11]. In European-Caucasian patients, a study using skin tape strip found that concentration of inflammatory cytokine in stratum corneum such as IL-18 CCL17, TARC/CCL17, MDC/CCL22, Cutaneous T-cell attracting chemokine (CTACK)/CCL27, IL-8/CXCL8 are high significantly in AD with skin lesions rather than in AD non lesions [12] Another study that using skin tape strip, found a group with 45 proteins as principal component 1 (PCI1) highest in AD with food allergy (FA). It has correlation with elevated trans-epidermal water loss (TEWL), specifically serine protease inhibitors b12 (SERPINB12) and gelsolin (GSN). 45 PCI1 proteins also significantly high in AD with FA rather than AD without FA and lowest in control group [13]. Studies found that there is characteristics in AD sample both blood or skin and can be identify the severity of AD (Table 1).
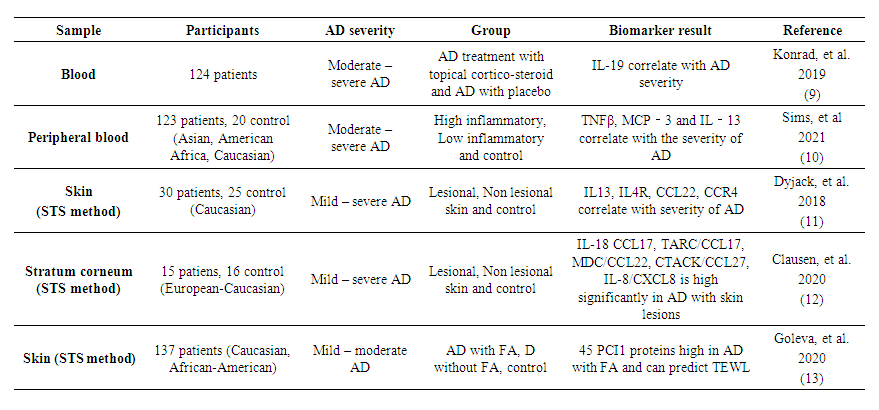 | Table 1. Characteristics and Severity of Atopic Dermatitis |
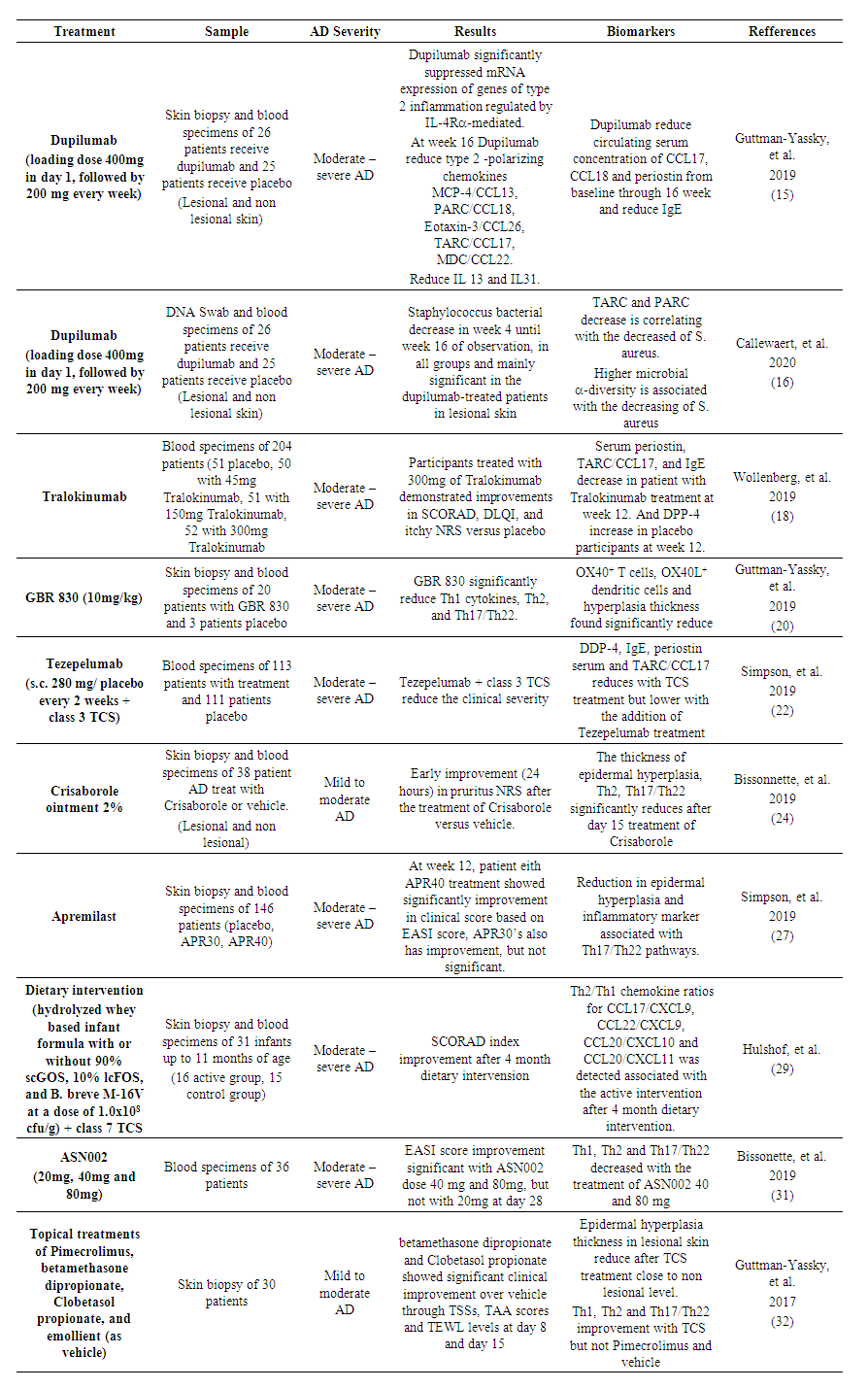 | Table 2. The role of biomarker in management of Atopic Dermatitis |
4. Conclusions
- In AD, clinical symptom assessment such as SCORAD index, EASI score, pruritus NRS, widely used worldwide. But the assessment is subjective. Biomarkers in AD play important role as objective assessment. Biomarkers also can help to breakdown the best personalized medicine for DA. It is still necessary to study the role of biomarkers to detect the safety and possibility of appropriate therapy in several conditions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML