-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2021; 10(1): 5-11
doi:10.5923/j.ajdv.20211001.02
Received: Aug. 8, 2021; Accepted: Aug. 20, 2021; Published: Aug. 25, 2021

Descriptive Study of the Clinical and Histopathological Features of Autoimmune Blistering Dermatoses
Hayder Alhamami1, Asmaa Al-jawad2, Ameer Dh Hameedi3
1Iraqi Board for Medical Specializations, Baghdad, Iraq
2Dermatology Center, Medical City, Baghdad, Iraq
3Department of Pathology, University of Baghdad, College of Medicine, Baghdad, Iraq
Correspondence to: Asmaa Al-jawad, Dermatology Center, Medical City, Baghdad, Iraq.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Autoimmune skin blistering diseases are a diverse group of dermatoses characterized by autoantibodies binding to antigens in the skin and mucous membranes. Some are associated with significant morbidity and mortality. Objectives: To study the clinical aspects, histopathological findings and immunofluorescence features of autoimmune blistering skin diseases. An attempt will be made to correlate the clinical with the histopathological features. Patients and Methods: This was a descriptive study conducted at the Center of Dermatology and Venereology, Baghdad Medical City, Baghdad, Iraq, from October 2018 to March 2020. A total of 60 patients were included. Diagnoses were established by history, clinical examinations, and skin biopsies with Hematoxylin and Eosin (H&E) staining. Direct immunofluorescence (DIF) tests were performed in some cases. Results: Thirty-five represented (58.3%) of the included patients were female, and 25 (41.6%) were male. The patients’ mean age was 49.4 ± 20.9 years. Bullous Pemphigoid was the most common disease (35% of the cases), followed by Pemphigus Vulgaris (31.6%). Pemphigus Vulgaris affected mostly middle-aged patients (mean age: 47 ± 16.66 years), whereas Bullous Pemphigoid affected mostly older patients (mean age: 60 ± 15.26 years). Bullae were the primary presented lesions (68.33% of the patients). Conclusion: Consistency between clinical diagnosis and histopathological results was found in 92% of the patients, suggested that careful history, clinical examination, and histopathological findings obtained from H&E-stained sections were sufficient to reach a diagnosis in most cases.
Keywords: Autoimmune vesiculobullous disorders, Pemphigus Vulgaris, Bullous Pemphigoid, Histopathology
Cite this paper: Hayder Alhamami, Asmaa Al-jawad, Ameer Dh Hameedi, Descriptive Study of the Clinical and Histopathological Features of Autoimmune Blistering Dermatoses, American Journal of Dermatology and Venereology, Vol. 10 No. 1, 2021, pp. 5-11. doi: 10.5923/j.ajdv.20211001.02.
Article Outline
1. Introduction
- Autoimmune skin blistering diseases are a diverse group of dermatoses characterized by autoantibodies binding to antigens in the skin and mucous membranes [1]. The etiopathogenesis of Pemphigus is characterized by the formation of autoantibodies directed against various proteins of the desmosomes. The binding of these autoantibodies to desmosomal components disrupts the intraepidermal adhesions, leading to acantholysis and intraepithelial blister formation [2,3]. Patients with Bullous Pemphigoid and other autoimmune subepidermal blistering diseases have autoantibodies that target autoantigens in the epidermal basement membrane [4]. The Pemphigoid group is characterized by subepidermal blisters filled with inflammatory cells, especially eosinophil [5]. We undertook this study to evaluate the clinical aspects, histopathological findings, and immunofluorescence features of autoimmune bullous dermatoses and to assess the correlation between clinical and histopathological features.
2. Materials Patients and Methods
- This was a descriptive clinical and histopathological study conducted at the Center of Dermatology and Venereology, Medical City, Baghdad, Iraq, from October 2018 to March 2020. All patients with suspected immunobullous diseases based on clinical history and examination undergoing skin biopsies for histopathological examination were included in the study. Patients with non–immune mediated bullous disorders secondary to mechanical injury, infections, eczemas, burns (chemical or thermal), and hereditary bullous disorders were excluded. According to the inclusion and exclusion criteria, 60 patients with autoimmune blistering dermatoses were included. The study was approved by the Scientific Council of Dermatology and Venereology, Iraqi Board for Medical Specialization. All participants were informed of the study’s aims and methods and provided written informed consent. A full history was taken from all patients, including age, duration of the disease, site of the lesions, associated symptoms, relevant family history, drug history, and any associated systemic diseases. Careful local and general examinations were performed to ascertain the presence or absence of intact bullae, whether the bullae were tense or flaccid, and to elicit Nikolsky’s sign and the Asboe-Hansen sign. The sites involved, skin erosions, and perilesional erythema were noted. Careful examinations of the oral mucosa, nails, eyes, and genitalia were also performed. Samples for histopathological examinations were collected from the lesional skin or the oral mucosa, placed in a 10% formalin solution, and sent to the laboratory. Slides were stained with Hematoxylin and Eosin (H&E) and examined under a light microscope (MCX100LCD; Micros Austria). Each slide was examined under ×40, ×100, and ×400 magnifications. Samples for direct immunofluorescence (DIF) were obtained from the perilesional skin. Frozen sections were cut and stained with antisera specific for immunoglobulin A (IgA), IgG, IgM, and C3. The patterns and distribution of immune complex deposits were analysed under a fluorescence microscope.
3. Results
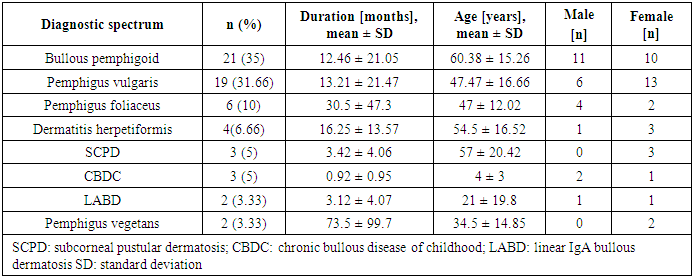
- Thirty-five patients represented (58.3%) of the included patients were females, and 25 (41.6%) were males. Bullous Pemphigoid was the most common disease, followed by Pemphigus Vulgaris. Pemphigus Vulgaris affected middle-aged patients, whereas Bullous Pemphigoid affected older patients. The demographic and clinical characteristics of the patients are shown in Table 1.
|
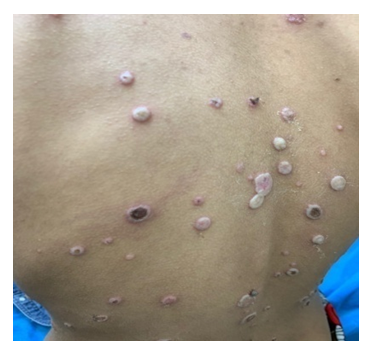 | Figure 1. Pemphigus vulgaris: flaccid blisters with erosion |
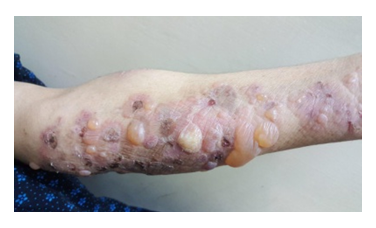 | Figure 2. Bullous Pemphigoid, tense blisters on an erythematous base |
|
|
|
|
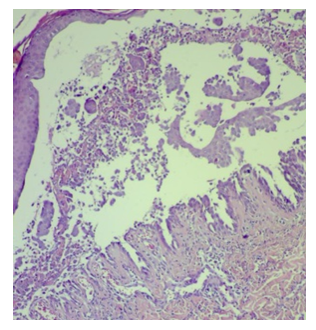 | Figure 3. Pemphigus Vulgaris, Intraepidermal blister x100 magnification |
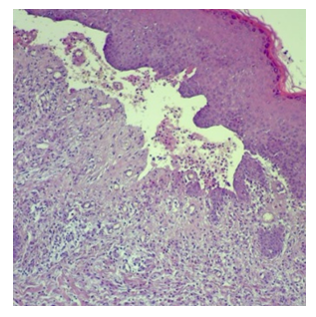 | Figure 4. Bullous Pemphigoid, subepidermal blister with eosinophilic infiltrate |
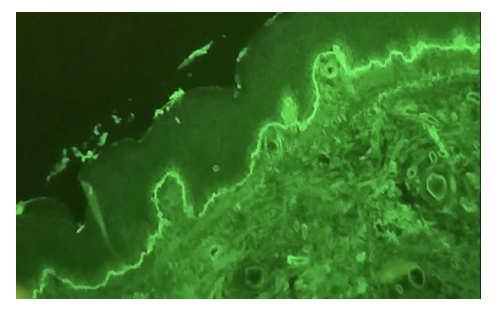 | Figure 5. Bullous Pemphigoid, DIF test with linear IgG along the basement membrane |
4. Discussion
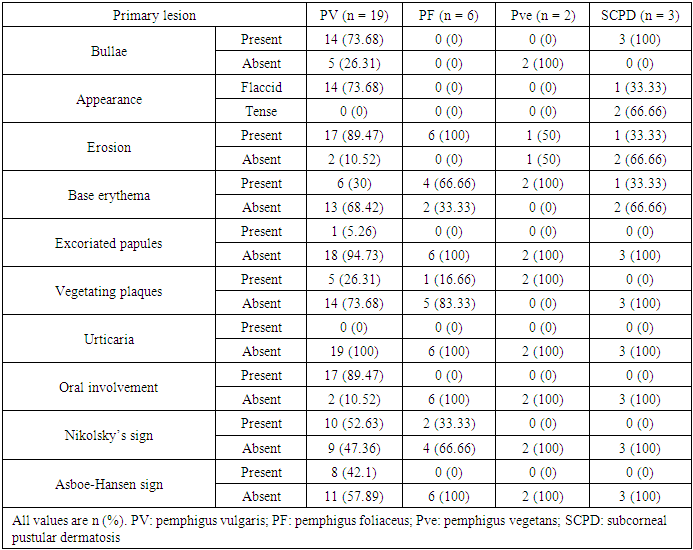
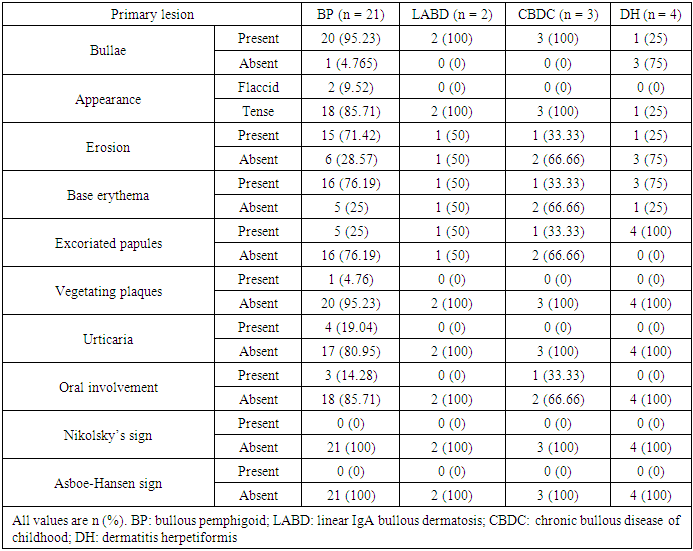
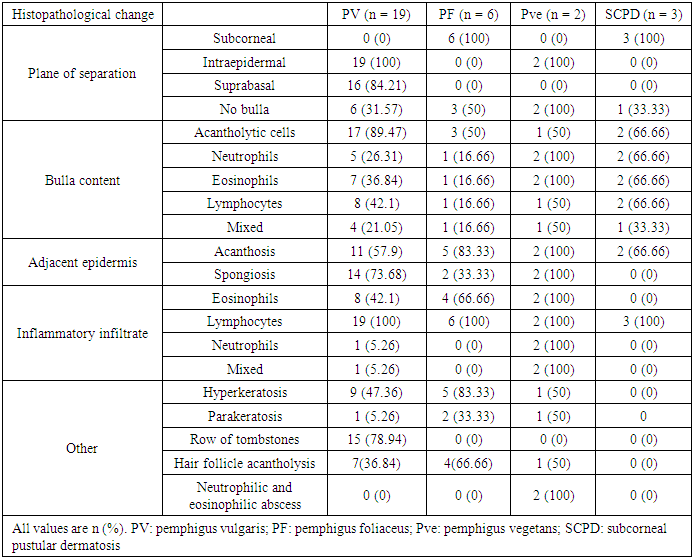
- Immunobullous diseases, are characterized by autoantibodies against adhesion molecules of the skin [6]. They manifest as fluid-filled cavities, which are termed vesicles or bullae, depending on their size. Light microscopy with H&E staining is the simplest method for the diagnosis and classification of immunobullous diseases [7]. Early diagnosis is important to prevent catastrophic complications. Although DIF is considered the gold standard, in resource-poor settings where it is not available, the diagnosis is based on clinical and histopathological findings only.In this study, BP was the most common immunobullous disorder, followed by PV. This is not in line with studies in neighbouring countries, such as Turkey, Iran, and Kuwait, which have found PV to be the most common autoimmune bullous disease, followed by BP [8-10]. Conversely, like in our study, a study conducted in India found BP to be the most common immunobullous disease [11]. These differences may be related to the referral policy, because our study is hospital based and does not reflect the true incidence in the community. In this study, BP affected mostly older patients (mean age: 60.38 years). This finding is consistent with Kutlubay et al. (75.6 years) and Mittal et al. (60 years) [10,12]. The mean age of presentation of PV in this study was 47.47 years, which is also in line with Kutlubay et al. and Mittal et al. [10,12]. By contrast, Nanda et al. reported a younger mean age (36.4 years) [8].The main lesions in PV patients in this study were flaccid bullae on an erythematous base. This finding is consistent with several previous studies [7,13,14]. All but two PV patients showed cutaneous involvement. The two differing patients presented with oral erosions without cutaneous manifestations. Oral involvement was noted in most of the patients, which were in line with other studies [12-14]. Nikolsky’s sign was positive in 52.63% of PV patients. Mittal et al. reported a comparable rate (49%) [12], whereas Deepti et al. and Kudligi et al. reported higher rates (88.2% and 93.5%, respectively) [13,14]. The Asboe-Hansen sign was present only in PV patients, at a rate of 42.1%, which is comparable to that reported by Deepti et al. (35.3%) but lower than that reported by Mittal et al. (93.5%) [12,14]. All PF patients in this study presented with erythematous skin erosions, except for one patient who presented with erosions and blisters. Kudligi et al. reported flaccid blisters and erosions covered with crusts in all PF patients [13]. The mucous membrane was spared in all patients. This was inconsistent with Deepti et al. who found oral mucosal involvement in 25% of their patients [14]. PF has only anti Desmoglein1 (Dsg1) antibodies; therefore, Desmoglein3 can compensate for the loss of Dsg1 in the mucosa preventing blister formation. Transition between the two Pemphigus types is a well-known phenomenon and this may explain the oral involvement described in some PF cases [15]. The main site of cutaneous involvement in the PF patients in our study was the trunk, which is consistent with Prakash et al. [7].Only two patients were diagnosed with PVe in this study. One of them had a 12-year history of pruritic erythematous vegetating plaques on both sides of the inguinal region, with no oral involvement. The other patient presented with erythematous plaques with erosions on both axillae and no other site involved. Patient was clinically suspected of having Hailey-Hailey disease; however, the histopathological findings showed PVe. Kudiligi et al. described PVe as flaccid blisters and vegetating skin lesions with non-erythematous bases [13]. Sharquei et al. described eight PVe patients presenting with well-defined erythematous verrucous and hypertrophic plaques of various sizes and durations with histological changes [16].All BP patients in this study complained of severe itching, which was also described by Kudligi et al. [13]. Deepti et al. reported itching in 84.6% of patients [14]. Oral involvement was noted in only 14% of patients, which is consistent with Mittal et al., Kudligi et al., and Deepti et al. [12-14]. Bullae were present in most of BP patients. Similarly, Deepeti et al. observed bullae in all their patients [14]. Nikolsky’s sign was negative in all BP patients, which was consistent with Kudligi et al. [13]. Conversely, Deepti et al. reported a rate of 53.8% [14].The main manifestation of DH in this study was excoriated papules, which were observed in all patients. Tense bullae were present in one patient (25%). These findings were in line with Kudligi et al., who also found excoriated papules in all DH cases [13]. Conversely, Deepti et al. reported pustules and tense bullae as the main manifestations (50% of the patients) [14]. The oral mucosa was not involved in any of study DH patients, which is consistent with Mittal et al. and Kudligi et al. [12,13].Two LABD cases were encountered in this study. Both presented with tense bullae with basal erythema. This was similar to the findings reported by Kudligi et al. [13]. No oral mucosal involvement was noted in studt patients, which was also in line with Kudligi et al. [13]. CBDC manifested as tense bullae in this study. Mittal et al. also described tense bullae in the only case in their study [12].Of the 19 PV patients in this study, clinicopathological consistency was seen in 18 patients. One patient was clinically suspected of having PF, but the histopathological findings were typical of PV. Suprabasal splits were noted in 84.2% of the patients, with rows of tombstones in 78.9%. This has also been described in other studies [7,12,14,]. Acantholytic cells were present in 89.5% of study patients, which is comparable to the rate reported by Basu et al. (79.41%) [17]. Hair follicle acantholysis was present in 36.8% of PV cases and 66.6% of PF cases. This was also described by See et al. [18]. Eosinophilic spongiosis, which has been described in the literature as an early finding in PV, was noted in 42.1% of PV patients. Prakash et al. also found Eosinophilic spongiosis in some cases and considered it a typical finding in early stages [7]. The main cell infiltrates within the blisters were lymphocytes in 42.1% of study cases, which was in line with Prakash et al. [7], but inconsistent with Basu et al. and Kudligi et al., who found neutrophils to be the most common cells [13,17]. The nature of the infiltrate is related more to the chronicity of the lesions.In this study, PVe exhibited histological findings of epidermal hyperplasia with neutrophils and Eosinophilic abscesses. Acantholytic cells were found in one patient. These findings are in line with Kudligi et al. [13]. Sharquei et al. described the histopathology of PVe as a suprabasal cleft with a thick strand of downward growth of epidermal hyperplasia into the dermis, giving rise to papillomatosis, with eosinophilic spongiosis and eosinophilic pustules in the epidermis [16].Subepidermal blisters were seen in 94.4% of BP patients, with osinophil being the main inflammatory cells. This is in line with Prakash et al and Deepti et al., who also reported eosinophil to be the main inflammatory cells [7,14]. Conversely, Kudligi et al. found neutrophils to be the main inflammatory cells [13]. Festooning was seen in eight (38.1%) cases in this study, which is comparable to the rate reported by Fatma et al. (21.42%) [19].LABD patients in this study showed subepidermal splits. The inflammatory infiltrates within the splits were mixed neutrophils, eosinophil, and lymphocytes. Kudligi et al. reported one case of LABD with subepidermal bullae with neutrophilic infiltration [13]. CBDC patients in this study showed subepidermal splits at a rate of 66.6%. Neutrophils were found in two patients. Mittal et al. described one CBDC patient in whom no bullae were found on histopathology, and the major inflammatory cells were neutrophils [12].In PV cases, DIF showed intercellular deposition of antibodies with positive IgG and negative C3. Mittal et al. described a lace-like squamous intercellular pattern with positive IgG [12]. In our study, BP showed linear deposition of IgG and C3 along the basement membrane, which is in line with Mittal et al. and Deepti et al. [12,14]. DIF findings were negative in five other patients, all of whom were on long-term systemic steroids at doses lower than 30 mg per day and presented with relapse. Judd and Lever found that high daily doses of steroids resulted in clinical improvement and showed a marked drop in the titer of circulating antibodies. Conversely, they found no predictable correlation between disease activity and antibody titer when patients were not receiving high doses of steroids [20].
5. Conclusions
- Consistency between the clinical diagnosis and the histopathological results was noted in 92% of study patients. This suggests that careful history, clinical examination, and histopathological findings obtained from H&E-stained sections were sufficient to reach a diagnosis in most cases. DIF is helpful if available.
Disclosure
- This study was an independent study and not funded by any of the drug companies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML