-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2021; 10(1): 1-4
doi:10.5923/j.ajdv.20211001.01
Received: Dec. 24, 2020; Accepted: Feb. 3, 2021; Published: Feb. 26, 2021

Terbinafine Comparative Study Conventional Dose versus Double Dose in Dermatophytosis Treatment
Abu Jafar Md. Shahidul Hoq
Cumilla Medical College Hospital, Cumilla, Bangladesh
Correspondence to: Abu Jafar Md. Shahidul Hoq, Cumilla Medical College Hospital, Cumilla, Bangladesh.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: Dermatophytosis is the most common fungal infections globally. Terbinafine is considered to have good potency against dermaphytes, but resistance to Terbinafine is on the rise. Objectives: The objective of this study was to do comparative study of Terbinafine in conventional dose versus double dose in treatment of Dermatophytosis. Methods: This cross-sectional comparison study conducted in the outpatient department of Cumilla Medical College Hospital, Cumilla, Bangladesh during the period from July 2018 to June 2019. In this randomized comparative study, patients of Tinea cruris and Tinea corporis were randomly divided into two groups of 87 each and were given oral Terbinafine in conventional dose (Group I) and oral Terbinafine in double dose (Group II) for 4 weeks. The scores and percentage changes measured from pruritus, scaling, and erythema were evaluated at 2nd and 4th weeks. Result: In total of 60 patients from both the groups completed the study. In this study it was found the Improvement rate of terbinafine double dose in higher 11.1 and 88.34 then the single dose. Conclusion: This study indicates that Terbinafine in a dose of 500 mg (double dose) given once daily was more effective than Terbinafine 250mg in the treatment of patients with Dermatophytosis.
Keywords: Terbinafine, Conventional Dose, Double dose, Dermatophytosis
Cite this paper: Abu Jafar Md. Shahidul Hoq, Terbinafine Comparative Study Conventional Dose versus Double Dose in Dermatophytosis Treatment, American Journal of Dermatology and Venereology, Vol. 10 No. 1, 2021, pp. 1-4. doi: 10.5923/j.ajdv.20211001.01.
Article Outline
1. Introduction
- Dermatophytic infections are the most common fungal infections affecting 20%–25% population globally. [1] The hot and humid climate in the tropical and subtropical countries like Bangladesh makes Dermatophytosis a is very common fungal infection. Terbinafine is considered to be a first-line drug for the treatment of dermatophytic infections (Tinea corporis and Tinea cruris) due to its favorable mycological and pharmacokinetic profile. [2] It acts by inhibiting the enzyme Squalene epoxidase, thereby inhibiting Ergosterol synthesis. [3] In the past, the drug was consistently effective against Dermatophytosis with cure rates of >90% achieved at doses of 250 mg once a day for 2 weeks. Recently, clinical failure and relapses have been observed with Terbinafine in patients with Tinea infections [4] with increase in incidence of Terbinafine resistance [5,6,7]. One of the principal mechanisms of antifungal resistance is a decrease in effective drug concentration. [6] This may include low plasma concentration [8] and incomplete cure [5]. Terbinafine was reported to be efficacious and safe in Dermatophytosis with fewer failure rates at higher doses of 500 mg/day for 4 weeks. [9] Hence, there was need for different treatment strategy while using Terbinafine. We conducted this survey with the aim of evaluating the conventional dose (250 mg) and double dose (500 mg) of Terbinafine in treatment of patients with Dermatophytosis.
2. Objectives
- a) General objective:• To compare between Terbinafine in conventional dose and double dose on the patients in treatment of Dermatophytosis.
3. Methodology & Materials
- This was a cross-sectional conducted in outpatient department of Cumilla Medical College Hospital, Cumilla, Bangladesh during the period from July 2018 to June 2019. In this randomized comparative study, patients of Dermatophytosis were randomly divided into two groups of 60 in total number and were given oral Terbinafine in conventional dose 250 mg as group I and oral Terbinafine in double dose 500 mg as group II for 4 weeks. The scores and percentage change in scores of pruritus, scaling, and erythema were evaluated at 2 and 4 weeks. The information was collected from doctor through observation and baseline parameters were collected from the patient records and files. Collected data was sorted and screened for any discrepancy. The edited data was entered on to the MS Excel 2016. For background variables and socio-demographic data descriptive statistics and relative frequency (percentage) was generated. In addition descriptive statistics and univariate statistics were generated to compare.
4. Result
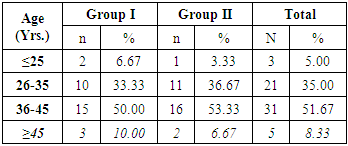
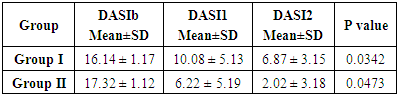
- There has been taken 60 patients for this study. Where 30 patients try Terbinafine 250mg and other 30 patients try 500mg. According to the age distribution of the two groups there the highest number, 31 (51.67%) was found in 36-45 years’ age group on that 15(50.00%) patients in group-I and 16(53.33%) in group-II. It was followed by 21 (35%) from 26-35 age group, where 10(33.33%) from group-I and 11(36.67%) from group-II. Again 5(8.33%) in ≥45 age group where 3(10.00%) from group-I and 2(6.67%) from group-II and 3(5.00%) in ≤25 where 2(6.67%) from group-I and one (3.33%) from group-II. (Table-1) The improvement rate of Terbinafine 250mg and 500mg showed in the study. On that the improvement rate of Terbinafine 500mg is better than Terbinafine 250mg. From the study after 2 weeks the improvement rate of group II (Terbinafine 500mg) was 11.2% and the other group I (Terbinafine 250mg) was 6.06%. Again after 4 weeks group II (Terbinafine 500mg) was 88.34% and the other group I (Terbinafine 250mg) was 57.43%. (Figure-1) Now Dermatophytosis Area Severity Index-comparison (DASI) within two groups was recorded as DASI baseline group I (16±1.17) and group II (17.32±1.12). P value of group I was 0.0342 and group II was 0.0473. (Table-2)
|
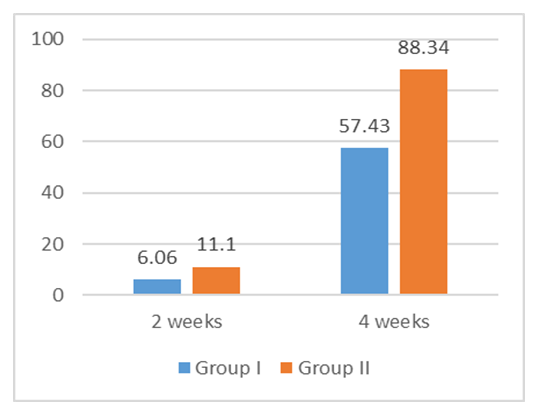 | Figure 1. Improvement rate of terbinafine 250mg and 500mg in both groups after 2 and 4 weeks (N=60) |
|
5. Discussion
- Widespread resistance to conventional doses of antifungals with increasing clinical failure rates warrants the search for an effective first-line antifungal drug that brings about rapid clinical and mycological cure in Tinea corporis and Tinea cruris. Terbinafine resistance when given in the standard conventional doses (250 mg once a day for 2 weeks) is being increasingly seen with partial or no response to treatment. [5] Antifungal resistance is due to a decrease in effective drug concentration because of extensive accumulation of Terbinafine in the skin and adipose tissue. [8] Hence, higher concentration of Terbinafine 500 mg/day has seen found to be more effective. [10,11,12]Although resistance to Terbinafine in Dermatophytosis is not common in clinical practice, it has been reported in clinical isolates by few authors. [5,6,7] Mukherjee et al. in 2003 reported first confirmed report of Terbinafine resistance in dermatophytes. [7] Majid et al. year? in their study reported that at the end of 12 weeks, there were only 43 cases out of the total 100 cases enrolled who were able to maintain a long-term clinical and mycological cure after 2 weeks of oral Terbinafine treatment. Authors concluded that incomplete mycological cure as well as relapse was very common after standard (2-week) Terbinafine therapy in patients of tinea cruris/corporis. [4] One of the principle mechanisms of antifungal resistance is decrease in effective drug concentration, [13] which in case of Terbinafine is quite known feature following standard dosing regimen of 250 mg daily due to extensive accumulation in skin and adipose tissue. [8] This clearly shows that the current standard Terbinafine therapy with 250 mg/day dose is not sufficient in current scenario where fungal resistance is further aggravated by increased use, inappropriate prescribing and over the counter sale of antifungal agents. [14,15]Even though there is no clear evidence as to what strategy should be used to best avoid resistance, the most commonly suggested measures in the past include prudent use of antifungals and appropriate dosing with special emphasis on avoiding treatment with low antifungal dosage. [16]Using higher dosages of Terbinafine thus seems useful strategy in countering these problems. Dolton et al. in their study reported that higher dose of Terbinafine was associated with highest trough and peak concentrations and area under the curve values in plasma which in turn were associated with lower minimum inhibitory concentration values and higher antifungal activity. We conducted this survey to find out the effectiveness of Terbinafine using higher dose and standard dose, 500 mg and 250mg once/day in patients with Dermatophytosis.There has been taken 60 patients for our study. Where 30 patients try Terbinafine 250mg and other 30 patients try 500mg. According to the age distribution of the two groups there the highest number, 31 (51.67%) was found in 36-45 years’ age group on that 15(50.00%) patients in group-I and 16(53.33%) in group-II. It was followed by 21 (35%) from 26-35 age group, where 10(33.33%) from group-I and 11(36.67%) from group-II. Again 5(8.33%) in ≥45 years age group where 3(10.00%) from group-I and 2(6.67%) from group-II and 3(5.00%) in ≤25 where 2(6.67%) from group-I and 1(3.33%) from group-II. The improvement rate of Terbinafine 250mg and 500mg showed in the study. On that the improvement rate of Terbinafine 500mg is better than Terbinafine 250mg. From the study after 2 weeks the improvement rate of group II (Terbinafine 500mg) was 11.2% and the other group I (Terbinafine 250mg) was 6.06%. Again after 4 weeks group II (Terbinafine 500mg) was 88.34% and the other group I (Terbinafine 250mg) was 57.43%. Now Dermatophytosis Area Severity Index-comparison (DASI) within two groups was recorded as DASI baseline group I (16±1.17) and group II (17.32±1.12). P value of group I was 0.0342 and group II was 0.0473. In our survey, Terbinafine given for 4 weeks was found to be most effective treatment strategy against superficial Dermatophytosis.The most common side effects with Terbinafine are gastric upset, headache, altered taste, altered liver function tests and rash; rarely, it can cause blood dyscrasias and hepatitis. [4] However, in our study, minor side effects such as nausea, bloating sensation, headache, and taste disturbances were seen. The results of our study suggest that Terbinafine 500mg is more successful than Terbinafine 250mg in fungal cure.
6. Limitations of the Study
- Our The study wasn’t a blinded study so patient bias was present along with observer bias in subjective recording and This survey has certain limitations because of the randomized comparative study, the possibility of selection bias cannot be ruled out. Long-term comparative studies to address the shortcomings of the present study are warranted.
7. Conclusions and Recommendations
- In this study, it was found that Terbinafine 500mg had higher clinical cure rates as compared to Terbinafine 250 mg. The failure rate was higher and the duration of treatment required is was longer. Even though that the resistance to Terbinafine was not common in clinical practice, its incidence was on the rise. Incomplete cure and relapses were very common with standard Terbinafine. The study indicated that Terbinafine in a dose of 500 mg as double dose given once daily was more efficacious than Terbinafine in a dose of 250 mg as conventional dose in the treatment of patients of Dermatophytosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML