Abdou Razak Moukaila1, 2, Panawè Kassang3, Komi Dzidzonu Nemi1, Kwame Doh4, Edem Komi Mossi1, Lidaw Déassoua Bawe2, Nouroudine Amadou1, Lihanimpo Djaloque5, Kokou Agbeko Djagadou1, Awalou Mohaman Djibril1
1Department of Internal Medicine, University of Lomé, Lomé, Togo
2Department of Infectious and Tropical Diseases, University of Lomé, Lomé, Togo
3Department of Dermatology, University of Lomé, Lomé, Togo
4Laboratory of Anatomopathology, University of Lomé, Lomé, Togo
5Department of Internal Medicine, University of Kara, Lomé, Togo
Correspondence to: Abdou Razak Moukaila, Department of Internal Medicine, University of Lomé, Lomé, Togo.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Primary Cutaneous Aspergillosis (PCA) is a relatively rare condition in people living with HIV (PLWHIV). Of the 25 cases reported in the literature, only one patient had advanced immunosuppression and none of the reported cases involved a black African subject. The objective of this work was to provide additional data to the scientific literature regarding cases of PCA in HIV- infected individuals. Observation: We report a case of PCA due to Aspergillosis Niger in a black PLWHIV subject who did not present any of the known factors associated with Aspergillosis, namely venous approach, neutropenia and trauma. The patient had an HIV1 / hepatitis C coinfection and consulted for finely scaly, pruritic, hyper-pigmented lesions forming a cupboard with a clear vesicular border in places that extended from the upper third of the trunk to the lower 2/3 of the thighs taking in the buttocks and external genitalia. The patient presented a good evolution of the lesions under treatment. Conclusion: Our observation describes a rare case of primary cutaneous aspergillosis in a subject immunosuppressed to HIV. Although rare in PLHIV, primary cutaneous aspergillosis, given the immunocompromised profile of patients, exposes them to systematic complications, potentially morbid. Hence the interest for the clinician to think of Primary Cutaneous Aspergillosis in the face of suggestive or atypical skin lesions for early diagnosis and management.
Keywords:
Cutaneous aspergillosis, Aspergillus Niger, HIV, Togo
Cite this paper: Abdou Razak Moukaila, Panawè Kassang, Komi Dzidzonu Nemi, Kwame Doh, Edem Komi Mossi, Lidaw Déassoua Bawe, Nouroudine Amadou, Lihanimpo Djaloque, Kokou Agbeko Djagadou, Awalou Mohaman Djibril, Atypical Form of Primary Cutaneous Aspergillosis in HIV1 Infected Individual, American Journal of Dermatology and Venereology, Vol. 9 No. 3, 2020, pp. 33-37. doi: 10.5923/j.ajdv.20200903.01.
1. Introduction
Immunosuppression due to HIV is associated with numerous opportunistic infections, among which mycotic infections play a significant role. The skin is one of the prominent organs affected by various ailments of which fungal infections occupy a privileged place. Aspergillosis are ubiquitous germs. While pulmonary and systemic aspergillosis remains widely described in the literature, this is not the case for primary cutaneous Aspergillosis, especially in people living with HIV (PLWHIV). The first case of cutaneous Aspergillosis in a PLWHIV was reported post-mortem in 1984 [1]. To date, the review of data in the literature between 1984 and 2012 regarding cases of primary cutaneous aspergillosis in PLWHIV has only enabled us to identify a total of 25 published cases (see Table 1) among which there were no cases involving a black subject [1–11]. We report a case of primary cutaneous Aspergillosis in a PLWHIV which caught our attention because of its clinical features. The purpose of this observation is to make an additional contribution regarding the primary cutaneous Aspergillosis in HIV immunocompromised subject, especially in black individuals.
2. Observation
This is a 53-year-old male subject, received in consultation in the Infectious Diseases Department of the Sylvanus Olympio University Hospital Center in Lomé, for pruritic dermatosis on HIV immunocompromised ground.Historically, the disease would have started about a year ago with pruritic papules that gradually evolved in a centrifugal manner invading almost the entire integument associated with persistent pruritus. The patient had HIV1 / hepatitis C co-infection and had been on antiretroviral therapy (Tenofovir / Lamividune / Efavierenz) for the past 07 months with an initial CD4 count of 435cel / mm3.He had no other known medical condition. The patient was self-reliant and had a stable general condition. Clinical examination of the skin revealed finely scaly, pruritic, hyper pigmented lesions with local excoriations and scratch marks, forming a cupboard with a clear vesicular border in places. This cupboard extended from the upper third of the trunk to the lower 2/3 of the thighs, taking the buttocks and external genitals without spaces of healthy skin (Figure 1).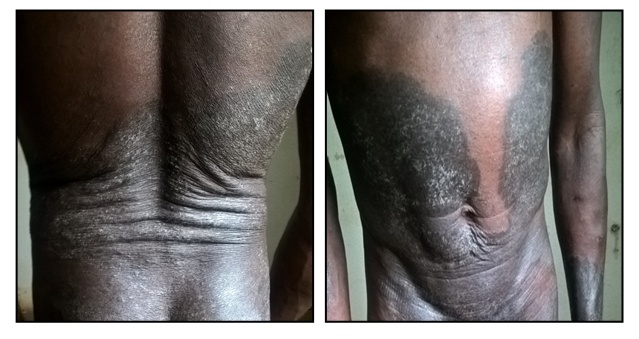 | Figure 1. Aspects of dermatological lesions |
Elsewhere, lesions with the same characteristics were found in the upper limbs but separated by small spaces of healthy skin.Examination of the mucous membranes and integuments was normal, as was the examination of the digestive, cardiovascular, pleuro-pulmonary and spleno-ganglionic systems. Faced with this clinical presentation, which a priori suggested a mycosis but was not typical of cutaneous candidiasis, a biopsy sample was taken for an anatomopathological analysis. The results of this examination revealed in the epidermis, a hyperkeratosis, orthokeratotic, Malpighi’s mucosal body being the site of a psoriasiform acanthosis. The analysis also noted spongiosis, sometimes with formation of a vesicle and exocytosis of polymorphous inflammatory cells, the papillary dermis being the site of a superficial, polymorphous peri-vascular inflammatory infiltrate, (Figure 2). Standard staining did not reveal any aspergillary filaments.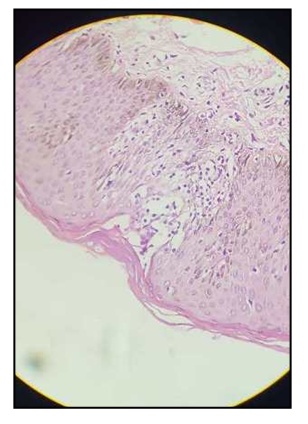 | Figure 2. Image of a histological section of the biopsy |
In the view of this histological aspect of a spongiotic and inflammatory dermatosis that may be part of a superficial cutaneous mycosis, a microbiological culture of a sample was carried out and allowed the isolation of a strain of Aspergillus Niger.The patient was offered treatment based on fluconazole 300 mg / week and ketoconazole in a foam bath solution. The evolution was already good after 3 weeks of treatment (Figure 3).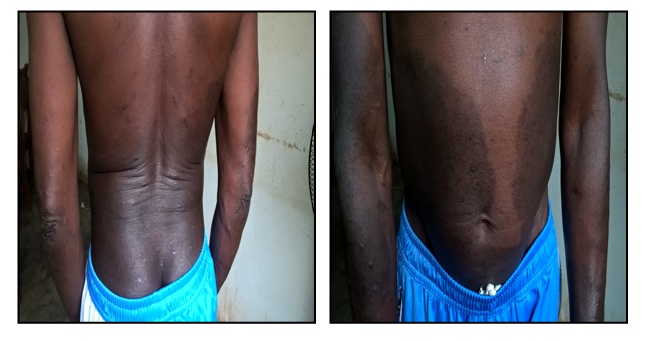 | Figure 3. Patient seen after three months of treatment |
3. Discussion
Aspergillus is ubiquitous germs. The first case of cutaneous Aspergillosis in a PLWHIV was reported in 1984 and was a post-mortem finding. Since then 24 cases of primary Aspergillosis affecting PLWHIV have been reported.Table 1 presents the characteristics of the 25 cases we were able to find in the literature. Primary cutaneous aspergillosis in PLWHIV appears to be the prerogative of patients with severe immunosuppression since out of 19 of the patients whose CD4 counts were reported, 18 had CD4 count below 200 cells / mm3.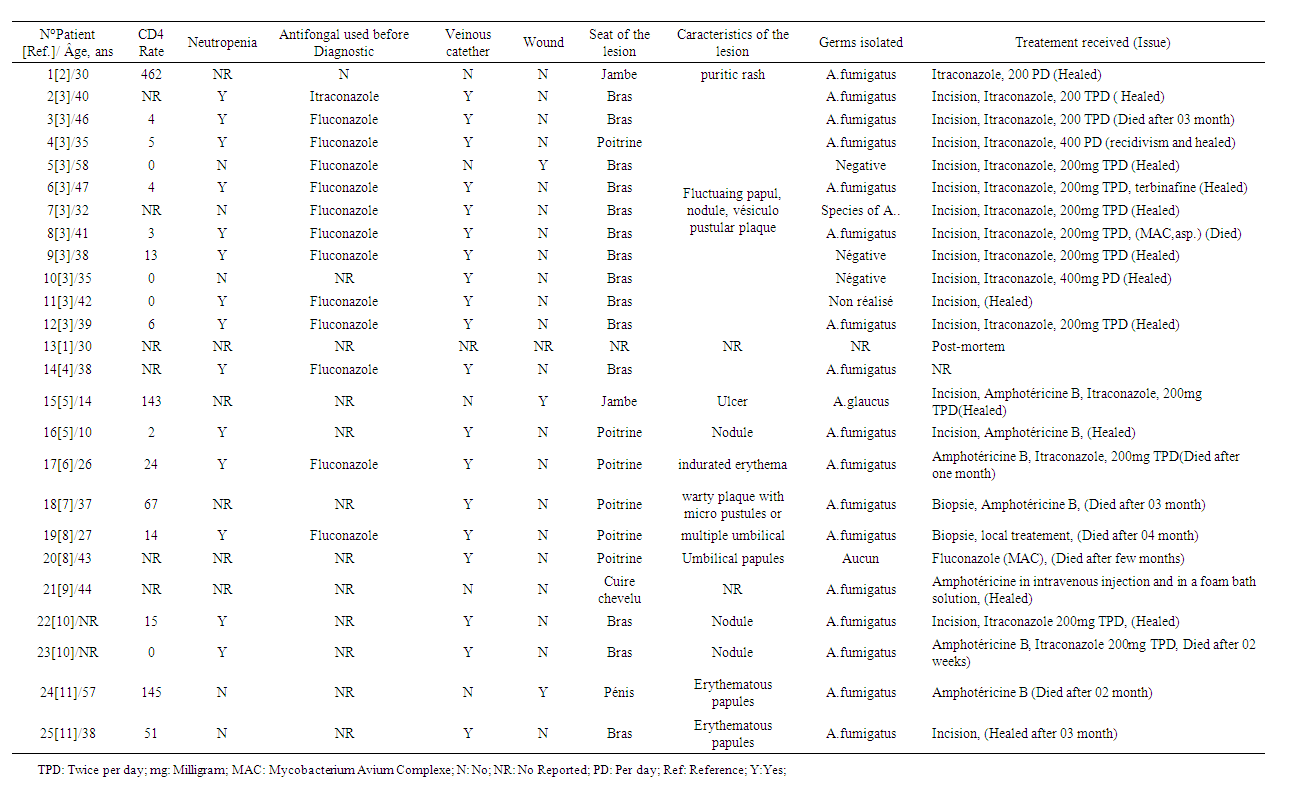 | Table 1. Characteristics of the cases of cutaneous Aspergillus in PLWHIV reported in the literature |
The presence of a venous catheter was reported in 79% of cases (19 cases out of 24). Neutropenia appears to be a factor in the occurrence of cutaneous Aspergillosis since certain ARVs such as Zidovudine and Ganciclovir have been proven to be myelotoxic, just as HIV itself is also thought to be involved in bone marrow dysfunction [12]. In previously reported cases, neutropenia was found in 14 of the 19 cases that reported neutropenia data. However, our case is similar to that reported by Guedela et al. in 2012 [2], who had neither a venous approach nor neutropenia but a CD4 count between 400 and 500 cel / mm3. The various lesions described in the literature in relation to primary cutaneous Aspergillosis in PLHIV included a pruritic rash, fluctuating papule, nodules, vesiculopustular plaques, indurated erythema, and warty plaque with micro pustules or multiple umbilical or erythematous papules.The singularity of the lesional extension of the case that we report deserves to be dwelled on since the atypical lesion presents in the form of a hyperpigmented, scaly and pruritic cupboard extending from the upper third of the trunk to the lower 2/3 of the thighs, taking the buttocks and external genitals without healthy skin spaces.Based on the history of the disease, we hypothesized that with regard to the initial lesions that were characterized by pruritus associated with papules, the patient would likely have presented prurigo, which is very common in black HIV immunocompromised individuals [13]. Prurigo scratching would have resulted in excoriations lesions that secondarily would have provided the gateway for Aspergillus conidia. The emancipation of these Aspergillus conidia would have been facilitated by the underlying immune system failure due to HIV but probably also to HCV. Indeed, conidiospores of Aspergillus circulate in the air and reach peak concentrations at seasonally variable times.An intact skin barrier provides effective protection against the penetration of conidiospores into the body. In the event of skin damage, Langerhans cells and other inflammatory cells (polynuclear neutrophils and monocytes) are capable of phagocytosis and intracellular destruction of the spores which manage to penetrate the deep layers of the skin [14].Thus, the causes of phagocyte cell dysfunction such as chronic granulomatous diseases, HIV, high-dose corticosteroid therapy, lead to an imbalance between lymphocytes T helper 1(Th1) and lymphocytes T helper 2 (Th2) with an excess of Th2 lymphocytes signaling pathways and may prevent the inhibition of inoculated spores promoting their germination into hyphae [14]. Specifically in people with HIV, a reduction in the antifungal activity of neutrophils has been described [15]. Similarly, a dysfunction of monocytes derived from macrophages has also been described in PLWHIV [16]. Another cause of this functional failure is the decrease in CD4 count associated with the synthesis of inhibitory factors by TCD8 lymphocytes [17].In addition, cytokine dysfunction with increased production and activity of cytokines including interleukin 10 appears to be related to decrease anti-Aspergillus activity of macrophages and neutrophils [18,19]. Furthermore, in subjects with chronic hepatitis C, the antigen presenting cells, the dentritic cells, in which the HCV seems to multiply, could also be involved in this functional failure of the immune response to Aspergillous invasion. Indeed, it has recently been shown that despite a normal phenotype and intact antigen-capturing capacity, the ability of dendritic cells to activate allogeneic T lymphocytes was profoundly impaired in patients with chronic hepatitis C in contrast to healthy subjects [20–22]. In addition, subjects with chronic hepatitis C also had an increased tendency to synthesize pro-inflammatory Th2 cytokines (IL-4, IL-5 and L-10) [23,24].All of these immunological phenomena could have contributed in addition to the decrease in HIV-related CD4 count, to the drastic decrease in the anti-aspergillary activity of the macrophages and neutrophils described above and thus to a real invasion of skin tissue by Aspergillosis in our patient.We isolated a strain of Aspergillus Niger from our patient to our knowledge, no case of Aspergillus Niger Aspergillosis on HIV immunocompromised ground had yet been reported. Indeed, in cultures carried out among the 24 previously reported cases, Aspergillus Fumigatus is by far the most isolated strain in 9 out of 10 cases. One case of Aspergillus Glaucus was reported by Shetty et al. in 1997 [5].Given the accessibility of the molecules in our context, we opted for a combination of oral Fluconazole combined with a topical treatment based on Ketoconazole with an evolution that was favorable for our patient with regression of the lesions. Among the cases reported in the literature, 13 cases were on antimycotic drugs prior to the diagnosis of cutaneous Aspergillosis of which 12 patients were already on Fluconazole and one patient on Itraconazole, suggesting resistance of Aspergillosis to these molecules.Nevertheless, it is essentially Itraconazole alone or in combination with Amphotericin B that has been used as a molecule in the care of subjects with a good evolution of the clinical picture in general. However, deaths occurring between two weeks and 12 weeks post- treatment have been reported depending on the case as a result of hematogenous dissemination of Aspergillosis either due to an underlying opportunistic infection such as Mycobacterium avium complex, Kaposi disease, toxoplasmosis., Cryptosporidiosis or leukemia.
4. Conclusions
The study observation describes a rare case of primary cutaneous Aspergillosis in an HIV immunocompromised subject. Skin biopsy and microbiological exploration allowed us to isolate a strain of Aspergillus Niger. This clinical case is of interest for two reasons. The first is the extensive nature that primary cutaneous Aspergillosis can take in people with HIV. The second is the interest of skin biopsy as the first option for exploring atypical skin lesions in PLHIV in order to guide the etiological diagnosis and ensure optimal patient care.
References
| [1] | Hui AN, Koss MN, Meyer PR. Necropsy findings in acquired immunodeficiency syndrome: a comparison of premortem diagnoses with postmortem findings. Hum Pathol 1984; 15: 670–6. https://doi.org/10.1016/s0046-8177(84)80293-2. |
| [2] | Gedela K, Nelson M, Francis N, Mohabeer M, Jones R. Cutaneous aspergillosis associated with HIV infection. Int J STD AIDS 2012; 23: 679–80. https://doi.org/10.1258/ijsa.2012.011437. |
| [3] | Murakawa GJ, Harvell JD, Lubitz P, Schnoll S, Lee S, Berger T. Cutaneous aspergillosis and acquired immunodeficiency syndrome. Arch Dermatol 2000; 136: 365–9. https://doi.org/10.1001/archderm.136.3.365. |
| [4] | Smith WF, Wallace MR. Cutaneous aspergillosis. Cutis 1997; 59: 138–40. |
| [5] | Shetty D, Giri N, Gonzalez CE, Pizzo PA, Walsh TJ. Invasive aspergillosis in human immunodeficiency virus-infected children. Pediatr Infect Dis J 1997; 16: 216–21. https://doi.org/10.1097/00006454-199702000-00010. |
| [6] | Girmenia C, Gastaldi R, Martino P. Catheter-related cutaneous aspergillosis complicated by fungemia and fatal pulmonary infection in an HIV-positive patient with acute lymphocytic leukemia. Eur J Clin Microbiol Infect Dis 1995; 14: 524–6. https://doi.org/10.1007/BF02113431. |
| [7] | Romero LS, Hunt SJ. Hickman Catheter-Associated Primary Cutaneous Aspergillosis in a Patient with the Acquired Immunodeficiency Syndrome. International Journal of Dermatology 1995; 34: 551–3. https://doi.org/10.1111/j.1365-4362.1995.tb02951.x. |
| [8] | Hunt SJ, Nagi C, Gross KG, Wong DS, Mathews WC. Primary cutaneous aspergillosis near central venous catheters in patients with the acquired immunodeficiency syndrome. Arch Dermatol 1992; 128: 1229–32. |
| [9] | Diamond HJ, Phelps RG, Gordon ML, Lambroza E, Namdari H, Bottone EJ. Combined Aspergillus and zygomycotic (Rhizopus) infection in a patient with acquired immunodeficiency syndrome: Presentation as inflammatory tinea capitis. Journal of the American Academy of Dermatology 1992; 26: 1017–8. https://doi.org/10.1016/S0190-9622(08)80347-7. |
| [10] | Van Burik J-AH, Colven R, Spach DH. Cutaneous Aspergillosis. J Clin Microbiol 1998; 36: 3115–21. |
| [11] | Arikan S, Uzun O, Cetinkaya Y, Kocagöz S, Akova M, Unal S. Primary cutaneous aspergillosis in human immunodeficiency virus-infected patients: two cases and review. Clin Infect Dis 1998; 27: 641–3. https://doi.org/10.1086/514694. |
| [12] | Dhurve SA. Bone Marrow Abnormalities in HIV Disease. Mediterr J Hematol Infect Dis 2013; 5. https://doi.org/10.4084/MJHID.2013.033. |
| [13] | Boozalis E, Tang O, Patel S, Semenov YR, Pereira MP, Stander S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol 2018; 79: 714-719.e3. https://doi.org/10.1016/j.jaad.2018.04.047. |
| [14] | Roilides E, Farmaki E. Human Immunodeficiency Virus Infection and Cutaneous Aspergillosis. Arch Dermatol 2000; 136: 412–4. https://doi.org/10.1001/archderm.136.3.412. |
| [15] | Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Impairment of Neutrophil Antifungal Activity against Hyphae of Aspergillus fumigatus in Children Infected with Human Immunodeficiency Virus. J Infect Dis 1993; 167: 905–11. https://doi.org/10.1093/infdis/167.4.905. |
| [16] | Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Defective Antifungal Activity of Monocyte-Derived Macrophages from Human Immunodeficiency Virus-Infected Children against Aspergillus fumigatus. The Journal of Infectious Diseases 1993; 168: 1562–5. |
| [17] | Clerici M, Roilides E, Via CS, Pizzo PA, Shearer GM. A factor from CD8 cells of human immunodeficiency virus-infected patients suppresses HLA self-restricted T helper cell responses. PNAS 1992; 89: 8424–8. https://doi.org/10.1073/pnas.89.18.8424. |
| [18] | Roilides E, Dimitriadou A, Kadiltsoglou I, Sein T, Karpouzas J, Pizzo PA, et al. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. The Journal of Immunology 1997; 158: 322–9. |
| [19] | Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, et al. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest 1994; 93: 768–75. https://doi.org/10.1172/JCI117031. |
| [20] | Bain C, Fatmi A, Zoulim F, Zarski J-P, Trepo C, Inchauspé G. Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C & Inchauspe GImpaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120: 512-524. Gastroenterology 2001; 120: 512–24. https://doi.org/10.1053/gast.2001.21212. |
| [21] | Akbar SM, Horiike N, Onji M, Hino O. Dendritic cells and chronic hepatitis virus carriers. Intervirology 2001; 44: 199–208. https://doi.org/10.1159/000050047. |
| [22] | Ito A, Kanto T, Kuzushita N, Tatsumi T, Sugimoto Y, Miyagi T, et al. Generation of hepatitis C virus-specific cytotoxic T lymphocytes from healthy individuals with peptide-pulsed dendritic cells. J Gastroenterol Hepatol 2001; 16: 309–16. https://doi.org/10.1046/j.1440-1746.2001.02383.x. |
| [23] | Tsai SL, Liaw YF, Chen MH, Huang CY, Kuo GC. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology 1997; 25: 449–58. https://doi.org/10.1002/hep.510250233. |
| [24] | Jacobson Brown PM, Neuman MG. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin Biochem 2001; 34: 167–71. https://doi.org/10.1016/s0009-9120(01)00210-7. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML