-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2020; 9(1): 11-16
doi:10.5923/j.ajdv.20200901.03

Ivermectin Cream is more Effective and Rapid than Permethrin Cream in Treatment of Patients with Scabies
Khalifa E. Sharquie1, Adil A. Noaimi1, Riyam A. Flayyih2
1Department of Dermatology, College of Medicine, University of Baghdad, Iraqi and Arab Board for Dermatology and Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
2Center of Dermatology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
Correspondence to: Riyam A. Flayyih, Center of Dermatology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Scabies is worldwide disease and it is one of the commonest causes of itching. Many therapeutic modalities for scabies are available, but none of them is uniformly effective 100% in treatment of scabies. Ivermectin cream had been used as an effective treatment of scabies. Objective: To assess the effectiveness of Ivermectin cream in comparison with Permethrin cream in the treatment of scabies. Patients and Methods: This study is a single, blinded, therapeutic comparative trial. It was conducted in the Center of Dermatology, Baghdad Teaching Hospital-Baghdad, Iraq during the period from April 2018 through October 2019. One hundred patients with scabies were enrolled in this work where all socio-demographic data that related to the disease were gained from each patient. History and clinical examination was done for each case to confirm diagnosis. The patients were divided into 2 groups according to the type of therapy: Permethrin group (A): Fifty patients treated by Permethrin cream 5%, 2.5% according to the age applied from the chin to toes at evening and washed after 12 hours and repeated after 7days. Ivermectin group (B): Fifty patients applied Ivermectin cream in the same manner of Permethrin. These patients were seen regularly every 2 weeks for 4 weeks duration, to re-evaluate for clearance of the disease and to record relapse and any local or systemic side effects. Results: At the end of four weeks of the study, the response to treatment was as follows: Permethrin (A): Response in 35 (70%) patients and no response in 15 (30%) patients Ivermectin (B): Response in 40 (80%) patients and no response in 10 (20%) patients. The result after two weeks in group A was not statistically significant but was statistically significant after four weeks. While in group B was statistically significant after two and four weeks after stopping therapy. So Ivermectin induced early cure when compared with Permethrin. The side effects were mainly mild burning sensation and irritation and this was noted in 7 (26%) patients in group A while in group B was 6(12%). While the recurrence rate was 6(12%) in group A and 5 (10%) patients in group B. Conclusion: Ivermectin and Permethrin cream were both statistically significantly effective in treatment of scabies but Ivermectin has quicker action after one week of therapy.
Keywords: Treatment, Scabies, Permethrin, Ivermectin Cream
Cite this paper: Khalifa E. Sharquie, Adil A. Noaimi, Riyam A. Flayyih, Ivermectin Cream is more Effective and Rapid than Permethrin Cream in Treatment of Patients with Scabies, American Journal of Dermatology and Venereology, Vol. 9 No. 1, 2020, pp. 11-16. doi: 10.5923/j.ajdv.20200901.03.
Article Outline
1. Introduction
- Scabies is one of the commonest endemic diseases in the world, particularly in the developing world [1]. The infection is endemic in many impoverished communities, but prevalence rates vary widely; seasonal outbreaks and documented peaks during times of war are probably related to crowding and population movements. [2] Scabies is endemic disease in Iraq since 1980s and from now and then there have been many outbreaks of this disease [3,4]. There are many therapeutic options that has been used in treatment of scabies like Gamma-Benzene-Hexachloride (Lindane 1%), Benzyl Benzoate 25%, Crotamiton 10%, Permethrin 5%, Malathion liquid (0.5%), Thiabendazole and Ivermectin cream 1%, most of them are differ in mechanism of action, duration of application, cost effectiveness, relapse and recurrence rate [5]. A 2019 meta-analysis reports that no single agent ranked effective with respect to cure and control of adverse effects, from the scabies infection. Permethrin and Pyrethrins were deemed most effective for cure and relief [6]. In (WHO)–sponsored study in the Solomon Islands, intervention of mass treatment with Ivermectin or Permethrin led to decrease in prevalence of scabies from 25% to less than 1%, as well as decrease in the prevalence of Pyoderma from 40% to 21%. [7]Permethrin: belongs to the Pyrethroid insecticide family, and contains Pyrethrum active ingredients and derivatives, formulated in a 5% cream that is currently, the standard topical scabicides. Like other Pyrethroid, it inhibits sodium transport in arthropod neurons, thereby causing paralysis. Permethrin is considered the most effective topical treatment for scabies [8] but it is associated with resistance, poor patient compliance, and allergic reactions. [9,10]. Permethrin 5% cream applied head to toe and washed off after 8-12 hours. The treatment must be repeated after 7-14 days. Permethrin is safe in pregnancy (category B) [11] and lactation [12,13] and is licensed for use in children from age of 2 months onwards. [14] Some patients may experience some irritation, burning sensation, but all are of short duration and most probably related to be applied on skin that is already sensitive, excoriated, and pruritic because of the scabies infection. Ivermectin is a macro cyclic lactone produced by Streptomyces avermitilis. Although not FDA-approved for scabies, Ivermectin represents an effective treatment for this and other ectoparasitic infestations. [15,16] By blocking transmission across nerve synapses that utilize glutamate or γ-aminobutyric acid (GABA), Ivermectin causes paralysis of peripheral motor function in insects and acarines. [15,16] Although GABA and glutamate are neurotransmitters within the human cerebral cortex, after early infancy, the blood–brain barrier prevents CNS penetration of the drug. Ivermectin is not recommended for children who weigh <33pounds (15 kg), pregnant women (category C), or breast feeding mothers. [17] Ivermectin is contraindicated in patients with an allergy to Ivermectin and CNS disorders. Topical Ivermectin in a 1% concentration appears to be effective for scabies, but further study is warranted before recommending its prevention/health promotion.For this reason this study was arranged to compare the effectiveness of Ivermectin cream to be compared with Permethrin cream in treatment of patients with scabies.
2. Patients and Methods
- This is a single, blinded, therapeutic, comparative study to compare the effectiveness of permethrin and ivermectin cream in treatment of scabies.One hundred and five patients with scabies were included in this study which was conducted in the Center of Dermatology and Venereology, Baghdad Teaching Hospital, Baghdad, Iraq during the period from April 2018 through October 2019. Five patients didn’t complete the follow up for transport difficulties and 100 patients with scabies completed this work. The diagnosis was established on clinical basis. Formal consent was taken from each contact persons after full explanation about the goal and nature of the present study. Also, ethical approval was given by the Scientific Council of Dermatology and Venereology-Arab Board for Medical Specializations.
2.1. The Diagnosis Based on
- • Presence of itching ranging from mild, moderate to severe (wakeup at nights).• Presence of characteristic pruritic papule, pustules and vesicles with or without excoriation.• Presence of one burrow or more.• Scraping of burrows for mite, eggs or scybala.• Presence of nodules.
2.2. Exclusion Criteria
- • Patients without burrows.• Recurrent attacks of scabies (for any reason).• Patients with chronic disease (renal failure, malignancy, uncontrolled diabetes mellitus, immunocompromized and other chronic disorder….etc.).• Patients less than one year of age.• Pregnant and lactating women.• Allergy to Ivermectin and Permethrin.• CNS disorders.All patients were interviewed and a full history was taken regarding: age, gender , occupation, residence, marital state, education, number of family members, duration of the disease, presence of itching and characterized by severe itching wakeup at night, history of previous treatment, positive family history of scabies, predisposing history like prison, travel, occupation, medical history (DM, other disease), drug intake.A clinical examination was carried out and scraping of burrows was done in most cases and to be seen under microscope looking for mites, eggs or scybala. Also the sites or distribution of burrows, characteristic pruritic papules, pustules, vesicles with or without excoriation and nodules were reported.Pre and post treatment photographs were taken using Samsung-digital, note 5 high sensitivity, 16 mega pixels, camera, in the same place with fixed illumination and distance.The patients were treated by either Permethrin cream or Ivermectin cream and were divided into 2 groups depending to the type of therapy:• Group A: Fifty patients treated by Permethrin cream 5%, 2.5% according to the age applied from the chin to toes at evening and washed after 12 hours and repeated after 7 days for one time by the same manner. Permethrin cream 2.5%: 30gm made in Jordan by Juman. Composition: Aqua, Stearic Acid, Glycerin, Cetyl Alcohol, Paraffinum Liquidum, Cetearyl Alcohol, Ceteareth-20, Glyceryl stearate, Permethrin, Propylene Glycol, Beeswax, Triethanolamine, Methylchloroisothiazolinone, Methylisothiazolinone.• Permethrin cream 5%: 30gm manufactured by Alpha Kozmetik Dezenfektan Urun. Made in Turkey.Composition: Aqua (deionized water), Permethrin, lanoline, Paraffinum Liquidum, Glycerin, Cetearyl Alcohol, Cetyl Alcohol, Stearic Acid, Glyceryl monostearate, Ceteareth-20, isopropyl myristatem, Phenoxyethanol, EDTA.• Group B: Fifty patients treated by Ivermectin cream was used as in the same way as in group A. Ivermectin cream 1% as Mectin® cream 30gram marketed by Forte Pharma Laboratories, made in France.Composition: Ivermectin BP 1% w/w, cream base q.s., excipients; Disodium EDTA, Methyl Paraben, Propyl Paraben, Propylene Glycol, Cetostearyl Alcohol, Cetonacrogol, Glyceryl Monostearate.
2.3. Follow up and Assessment of Drug Effectiveness
- The patients were seen regularly every two weeks for four weeks after stopping treatments. At each visit the response to treatment was assessed according to the change in itching severity especially wakeup at nights and disappearance of burrows, characteristic pruritic (papules, pustules, vesicles), excoriation and nodules disappearance or new nodules appearance. Any sign or symptom of local or systemic side effects of treatment modalities was reported. Assessment of drug effectiveness was based on the disappearance of burrows, characteristic pruritic papules, pustules, vesicles, excoriation and reduction of itching from sever, moderate, mild to nil. Nodules might be still present or reduction in size and number. So the response to therapy was decided as follow:• Response; disappearance of burrows, characteristic pruritic papules, pustules, vesicles and reduction of itching from severe to moderate, mild and nil, occurs in the 1st two weeks after stopping treatments and much better in the next two weeks.• No response; in patients had one burrow or more, characteristic pruritic papules, pustules, vesicle and excoriation still present or might be not, itching ranging from mild to severe with much worse in the next two weeks.• Recurrence or relapse; occurs in patient presented with full response to treatment then after had relapse in a form of one burrow or more with characteristic pruritic papules, pustules and vesicles, itching ranging from mild, moderate to severe (wakeup at night) and the patients had more sign and symptoms in the next weeks.
2.4. Instructions for Application
- Proper application must be achieved including the umbilicus, genitalia, up to the edge of all body orifices, and under the free edges of nails (nails are clipped) after one hour from bathing and any area of the body wash with soap must repainted with topical Permethrin or Ivermectin cream, and patients cloths must be boiled or laundered including the underwear. Treatment of clothes, fomites, and households were also recommended.
2.5. Complication of Disease and Treatments
- All patients were monitored for skin infection like impetignization, fever and lymph node enlargement or another complication of Pyoderma. Also patients were watched for post scabietic itching eczematous dermatitis, scabietic nodules, psychological complications and post-streptococcal glomerulonephritis.The side effects of therapy like presence of irritation and burning sensation after treatment were recorded.
2.6. Statistical Analysis
- Descriptive statistics (Measures of central tendencies and dispersion; like mean and standard deviation) were used together with analytic statistics (Chi-square) using EPI-Info version 20.
3. Results
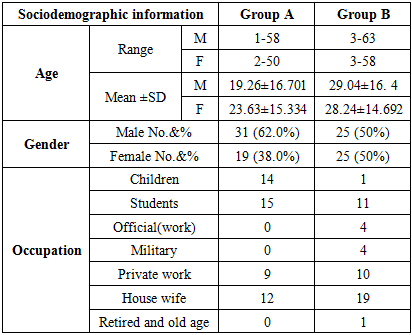
- A total of 100 patients who completed this study, 56 males (56%) and 44 females (%44), their ages ranged between 1-63 years with a mean ± SD of 22.42 ±15.667 years.
3.1. Clinical Data of the Two Groups at Time of Presentation before Treatment
- Group A: These patients 31 (62%) males and 19 (38%) females with a male to female ratio 1.63: 1; the age range of males at presentation was from 1-58 years with a mean ± SD of 19.26± 16.701 years; while the age range of females at presentation was from 2-50 years with a mean ± SD of 23.63 ±15.334 years. The disease duration ranged from 1-8 weeks with a mean ± SD of 3.12± 2.027 weeks.Itching was reported in all 50 patients which varied in severity from 1 (2%) patient to moderate and severe (wakeup at night) in 49 (98%) of patients. Family history of scabies was positive in 41 (82%) patients. Burrows found in 50 (100%) patients, characteristic pruritic papules, pustules and vesicles with or without excoriation were found in 46 (92%) while nodules were noticed in 28 (56%).
|
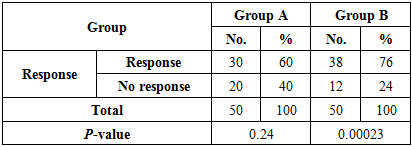
3.2. The Response to Treatment of Two Groups after Two Weeks of Stopping Therapy
- Group A:Response; was noted in 30 (60%) patients with a reduction in severity of itching to moderate 22 (73%) and mild 8 (27%) patients, disappearance of burrows and characteristic pruritic (papules, pustules and vesicles) in all these patients 30 (60%), while nodules were found in 13 (43%), not present in 17 (57%) patients. Irritation was noticeable in 5 (16%).No response; was noted in 20 (40%) patients with severe itching 15 (75%), moderate 5 (25%) patients; disappearance of burrows in 2 (10%) patients and found in 18 (90%) patients, while characteristic pruritic (papules, pustules and vesicles) found in all these patients 20 (40%), nodules were found in 11 (55%), not present in 9 (45%) patients. There was statistically insignificant Chi-square (χ2) = 1.34 and P-value = 0.24.Side effects; dermatitis was detected in 7 (26%) patients.Group B:Response; was noted in 38 (76%) patients with a reduction in severity of itching to moderate 14 (37%) and mild 24 (63%) patients, disappearance of burrows and characteristic pruritic (papules, pustules and vesicles) in all these patients 38(76%), while nodules were found in 16 (42%), not present in 22 (58%) patients. Burning sensation was noted in 2(%).
|
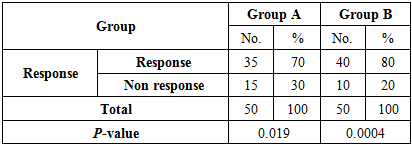
3.3. The Response to Treatment of Two Groups after 4 Weeks of Stopping Therapy
- Group A:Response; was noted in 35 (70%) patients with a reduction in severity of itching to moderate 5 (14%), mild 10 (29%) and nil 20 (57%) patients; disappearance of burrows and characteristic pruritic (papules, pustules and vesicles) in all these patients 35 (70%), while nodules were found in 4 (11%), not present in 31 (89%).No response; was noted in 15 (30%) patients with severe itching 8 (53%), moderate 5 (33%) and mild 2 (14%) patients; disappearance of burrows in 5 (33%) and found in 10 (67%) patients, while characteristic pruritic (papules, pustules and vesicles) found in all these patients 15 (30%), nodules were found in 6 (40%), not present in 9(60%) patients.Recurrence or relapse; occurred in 6 (12%) patients.There was statistically significant Chi-square (χ2) =5.43 and P-value P=0.019.Group B:Response; was noted in 40 (80%) patients with a reduction in severity of itching to moderate 3(7%), mild 12 (30%) and nil in 25 (63%) patients; disappearance of burrows and characteristic pruritic (papules, pustules and vesicles) in all these patients 40 (80%), while nodules were found in 12 (30%), not present in 28 (70%) patients.No response; was noted in 10 (20%) patients with severe itching 8 (80%) and moderate itching 2 (20%) patient; disappearance of burrows in 4 (40%) and found in 6 (60%) patients, while characteristic pruritic (papules, pustules and vesicles) found in all these patients 10 (20%), nodules were found in 3 (30%), not present in 7 (70%) patients.Recurrence or relapse; occurred in 5 (10%) patients.There was statistically significant Chi-square (χ2) = 12 and P-value P = 0.0004.
|
4. Discussion
- Scabies is one of the commonest endemic diseases in the world, particularly in the developing world [1]. There are many therapeutic options that has been used in treatment of scabies like Gamma-Benzene-Hexachloride (Lindane 1%), benzyl Benzoate 25%, Crotamiton 10%, %, Permethrin 5%, Malathion liquid (0.5%), Thiabendazole and Ivermectin cream 1% which differ in mechanism of action, duration of application, cost, relapse and recurrence rate [5]. To the best of our knowledge this is the first study in Iraq has been done to assess and evaluate the effectiveness of Permethrin and Ivermectin cream in treatment of scabies. In the present study, considering the cure rate of topical Ivermectin was 76% at end of 2 weeks while was 80% a 4 weeks and work was closely comparable with Goldust et al [18] where the cure rate was 65.8% of patients at the 2-week follow-up and 89.5% at the 4-week follow-up period [18]. Side effects of topical Ivermectin in this work were mild burning sensation and irritation in 6 (12%) patients in comparison with 6.9% (20/291) according to a systematic review and meta-analysis of randomized controlled [19]. The present study showed recurrence or relapse rate of topical Ivermectin was 5 (10%) in comparison with 10% (30/291), [19]. According to the cost of therapy, the present work showed that topical Ivermectin was more expensive than others. In comparison the efficacy of topical Ivermectin with other drugs of scabies, Goldust et al [20] showed that two applications of Ivermectin are as effective as single applications of Crotamiton 10% cream at the 2-week follow-up. After repeating the treatment, Ivermectin is superior to Crotamiton cream 10% at the 4-week follow-up [20]. Goldust et al [21] showed that two application of Ivermectin was as effective as single applications of Malathion 0.5% lotion at the 2- week follow-up. After repeating the treatment, Ivermectin was superior to Malathion 0.5% lotion at the 4-week follow up [21], but using of Malathion is forbidden nowadays.The present work showed the cure rate of Permethrin was 60% at 2 weeks of follow up and 70% at 4 weeks of study in comparison with the cure rate after one application ranges 89–98% while other reports showed that two applications 1 week apart has a cure rate of between 85% and 100%. [19,22,23]. Permethrin belongs to the Pyrethroid insecticide family, and contains pyrethrum active ingredients and derivatives, as there is little percutaneous absorption; there is low toxicity to humans. Also this drug rapidly degrades after taking effect, so it has no effect on the environment [6,11]. According to adverse effects of Permethrin, in the present study were mild burning sensation and irritation which are the only side effects 7(26%) which is comparable with other literature that showed 1-10% mild and transient burning and stinging (10%), pruritus (7%) [6,11]. But these side effects are much higher when compared with 4.6% (35/758) according to a systematic review and meta-analysis of randomized controlled trials [19]. The present work showed recurrence or relapse rate of Permethrin was 6 (12%) patients in comparison with 7% (20/289) by other studies [19]. Regarding the cost effectiveness, the present work recorded that Permethrin was non costly [24]. It has been reported that the efficacy of Permethrin in treating scabies is better than Ivermectin, Lindane, Benzyl Benzoate, Crotamiton and sulfur [19,22,23]. But this study demonstrated that topical Ivermectin is more effective than Permethrin cream at end of 2 and 4 weeks of therapy. Still other study showed that topical Ivermectin may have a similar efficacy to topical Permethrin depending on systematic review and meta-analysis of randomized controlled trial [19].The present work also showed that in 60%-76% of patients might need one single application of Permethrin or Ivermectin and no need to be repeated after one week.Sulfur 7% ointment is widely used in Iraq to treat scabies with three night's application. It is well tolerated by all ages including newly born infants. It gives a high cure rate around 90.6% with no important side effects when used for only 3 nights and non-costly therapy. Also sulfur seems to kill both mites and eggs as no need to be repeated after one week while most other topical therapies need to be repeated after one week in order to kill new mites hatching from eggs. [25]So in conclusion, Ivermectin and Permethrin cream were statistically significantly effective in treatment of scabies after four weeks of therapy but Ivermectin has quicker action than Permethrin cream after two weeks of stopping treatment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML