-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2019; 8(6): 95-98
doi:10.5923/j.ajdv.20190806.01

Methotrexate in Low Split dose as a First Line Treatment Option in the Management of Early Mycosis Fungoides
Khalifa E. Sharquie1, Fatema A. Al-Jaralla2
1Department of Dermatology, College of Medicine, University of Baghdad. Iraqi and Arab Board for Dermatology & Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
2Department of Dermatology, College of Medicine, University of Baghdad, Baghdad, Iraq
Correspondence to: Khalifa E. Sharquie, Department of Dermatology, College of Medicine, University of Baghdad. Iraqi and Arab Board for Dermatology & Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Cutaneous T-cell lymphomas (CTCLs) are a group of extranodal non-Hodgkin lymphomas that account approximately for 2% of all lymphomas. Treatment is based mainly on the recent published European Organization for Research and Treatment of Cancer guidelines due to the rarity of randomized studies. Basically, early-stage MF is treated with skin-directed treatments, whereas advanced-stage MF requires more aggressive therapies. Up to this date, low dose methotrexate is considered as a second line treatment. Objective: to test the effectiveness of split low dose methotrexate as a first line therapy for patch and plaque stage mycosis fungoides as its more available and with relatively few side effects. Patients and methods: This is an interventional study that was conducted in department of dermatology, college of medicine, university of Baghdad, Baghdad teaching hospital during the period from 2006 through 2019; 40 cases of MF in the patch and plaque stages of the disease were treated with methotrexate. All patients were diagnosed based on results of histopathology and clinical examination according to TNMB WHO classification updated in 2016 (stage MF IA: 17; MF IB: 23). Laboratory tests were carried out in all patients, including blood cell-count, blood levels of creatinine, urea, liver enzymes, and lactate dehydrogenase (LDH). Laboratory tests were repeated at monthly intervals during the treatment. All patients received oral MTX as 7.5 mg every three days, 10 mg prednisolone per day, topical steroids and systemic antihistamines for one month. Then patients were maintained with only MTX 7.5 mg every three days for 3-6 months after achieving remission. Resuls: Forty cases with early mycosis fungoides were treated, their ages ranged from 30-70 years with a mean of 50 years, 34 males and 6 females with a ratio 5.7:1. All patients responded quickly to this therapy regime and almost complete response was seen after one month. Patients were maintained on methotrexate only for 3-6 months and showed full remission with no noticeable clinical or laboratory complications. Conclusion: The present work had shown that methotrexate can be given safely as a first line therapy for patch and plaque stage mycosis fungoides with excellent results and a relatively few side effects. Methotrexate had shown longer remission periods with Sharquie regimen thus probably having prophylactic action in addition to its therapeutic effect; hence it might prevent the progression of mycosis fungoides.
Keywords: Methotrexate, Mycosis fungoides
Cite this paper: Khalifa E. Sharquie, Fatema A. Al-Jaralla, Methotrexate in Low Split dose as a First Line Treatment Option in the Management of Early Mycosis Fungoides, American Journal of Dermatology and Venereology, Vol. 8 No. 6, 2019, pp. 95-98. doi: 10.5923/j.ajdv.20190806.01.
Article Outline
1. Introduction
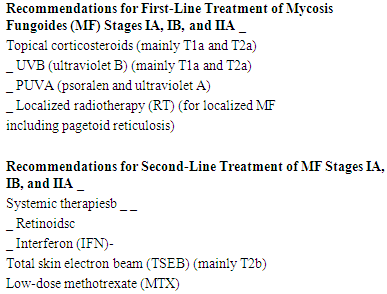
- Mycosis fungoides (MF) is the most common type of cutaneous T cell lymphoma (CTCL). It is defined by the cutaneous presentation and accumulation of a T helper memory/effector subset with a CD4+, CD8–, CD45RO+CLA+ phenotype. [1] Classic mycosis fungoides presents with 3 stages: patch (atrophic or nonatrophic), plaque, and tumor. Often, the first stage goes on for many years and might present with nonspecific dermatitis or poikeloderma like, which usually consists of patches and is often found on the lower trunk and buttocks. [2,3]The staging of MF is based on the particular skin findings and findings of extracutaneous disease that are observed in affected patients, with the stages defined as follows:• Stage IA disease (as defined by the tumor, node, metastases, blood [TNMB] system) - Patchy or plaque-like skin disease involving less than 10% of the skin surface area (T1 skin disease)• Stage IB - Patchy or plaque like skin disease involving 10% or more of the skin surface area (T2 skin disease)• Stage II (T1, T2) (N1, N2) N1 is palpable nodes without clear histologic evidence of lymphoma; N2 is palpable nodes, histologic evidence of lymphoma, node architecture preserved • Stage IIB disease - Tumors are present (T3 skin lesions)• Stage III disease - Generalized erythroderma is present• Stage IVA1 disease - Erythroderma and significant blood involvement occur • Stage IVA2 disease - Lymph node biopsy result shows total effacement by atypical cells (LN4 node)• Stage IVB disease - Visceral involvement (eg, liver, lung, bone marrow) occurs [3]Methotrexate (MTX) is an antimetabolite of the antifolate type. It competitively inhibits dihydrofolate reductase (DHFR) enzyme with an affinity of about 1000-fold that of folate. DHFR catalyses the conversion of dihydrofolate to the active tetrahydrofolate. Folic acid is needed for the de novo synthesis of the nucleoside thymidine, required for DNA synthesis. Also, folate is essential for purine and pyrimidine base biosynthesis, so synthesis will be inhibited. Methotrexate, therefore, inhibits the synthesis of DNA, RNA, thymidylates, and proteins. [4]The first report using MTX in MF was published by Wright in 1964; 9 out of 16 patients with MF achieved remission. MTX dose was 2.5 - 10 mg per weeks [5] Few studies followed afterwards concerning the effectiveness of MTX in treating different stages of MF; McDonald and Bertino reported the complete remission in 7 out of 11 patients with MF at stage II-III. [6] Zackheim et al confirmed positive results of low dose MTX in the treatment of erythrodermic CTCL. Complete remission was achieved in 41% and partial remission in 17% of treated patients. [7] Another study in 2003 performed on 69 patients with MF, 60% of patients had patch/plaque stage T2 disease (10% skin involved). Of these, 7 (12%) achieved complete remission and 13 (22%) achieved partial remission for a total response rate of 20 of 60 (33%). The median time to treatment failure was 15 months. Only 1 of 7 patients with tumor stage disease responded. [8]K. Olek-Hrab et al revealed that 56 patients out of 79 (70,9%) had achieved remission on the MTX. Patient staging were (stage MF I: 39; MF II: 16; MF III: 16; MF IV: 8). The remission began in the 1st month of therapy in 20% of patients, lasted 4 to 6 months in 50% of cases. At least 12 months’ remission was confirmed in 25% of patients (2-year-long only in 10% and 3-year-long in 5% of patients). The time to remission was related to the stage of disease at diagnosis as well as to minimal and maximal dose of MTX. The total therapeutic dose of MTX was found important for the course of the disease: higher total dose had prolonged the remission. [9]The National Comprehensive Cancer Network, the EORTC, and the European Medical Society of Oncology have developed treatment guidelines that might be helpful in guiding therapeutic strategies. [10]
|
2. Patients and Methods
- This is an interventional study that was conducted in department of dermatology, college of medicine, university of Baghdad, Baghdad teaching hospital during the period from 2006 through 2019; 40 cases of MF in the patch and plaque stages of the disease were treated with MTX. All patients were diagnosed based on results of histopathology and clinical examination according to TNMB WHO classification updated in 2016 (stage MF IA: 17; MF IB: 23). Laboratory tests were carried out in all patients, including blood cell-count, blood levels of creatinine, urea, liver enzymes, and lactate dehydrogenase (LDH). Laboratory tests were repeated at monthly intervals during the treatment. All patients received oral MTX as 7.5 mg every three days (2.5mg tablet three times a day), 10 mg prednisolone per day, topical steroids and systemic antihistamines for one month. Then patients were maintained with only MTX 7.5 mg every three days for 3-6 months after achieving remission.
3. Results
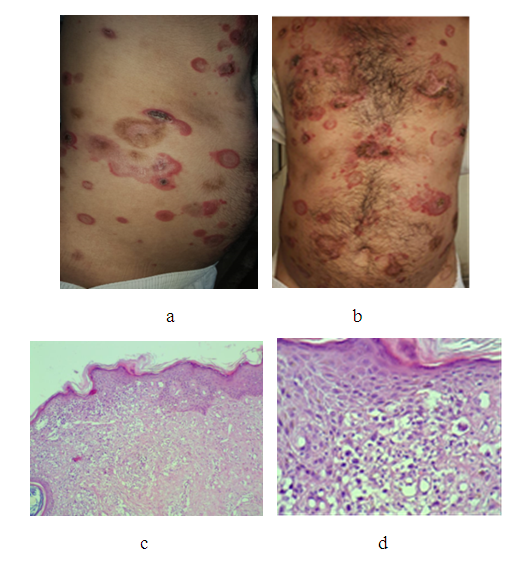
- Forty cases with early mycosis fungoides were treated, their ages ranged from 30-70 years with a mean 50years, 34(85%) males and 6(15%) females with a ratio 5.7:1. All patients showed quick response to this treatment regime and almost complete response was achieved after one month. Patients then were maintained on methotrexate only, for 3-6 months and showed full remission. Lesions had left post-inflammatory hyperpigmentation. No important clinical and laboratory complications and adverse were recorded. Figure 1 and 2
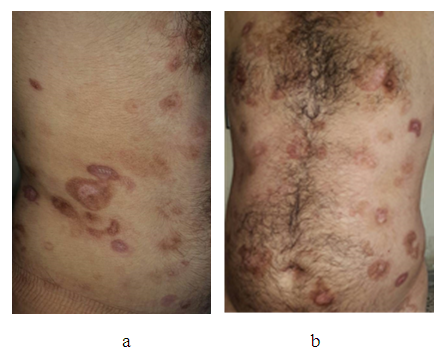 | Figure 2. (a and b) same patient in figure 1 after only 3 weeks of treatment showing dramatic response |
4. Discussion
- Cutaneous T-cell lymphomas (CTCLs) are a group of extranodal non-Hodgkin lymphomas that account approximately for 2% of all lymphomas. It is increasing all over the world but especially in Iraqi population following gulf war 1991 [11,12]. There are many modes of therapy for early cases of mycosis fungoides but basically, early-stage MF is treated with skin-directed treatments, whereas advanced-stage MF requires more aggressive therapies. Among the skin-directed therapies; UVB phototherapy, PUVA phototherapy, topical corticosteroids, topical chlormethine, topical retinoids (eg, bexarotene), and radiation (eg, external beam radiotherapy and total skin electron beam therapy) whereas low dose methotrexate is considered as a second line treatment. There are limited numbers of reports on the effectiveness of MTX in patients with MF. MTX is usually administered in the oral dose form as the second-line drug according to EORTC and WHO recommendations for stages IA-IIB. MTX should be given in a dose of 20 to 75 mg per week, usually divided in three doses every 12 hours weekly. MTX can also be combined with glucocorticoids, PUVA or INF-α. [9]Previous reports used methotrexate as single weekly dose either in oral or SC forms and ranging from 7.5 to 25 mg while Weinstein regimen using methotrexate in three divided doses over a 24-hour period each week. This split dosing decreases the chances of gastrointestinal upset. Most recently Sharquie et al 2019 used new split regime in treatment of psoriasis by using oral methotrexate 7.5mg every three days (one tablet 2.5mg three times a day) [13]. This new regime was more tolerated by patients with low gastrointestinal side effects and less hepatic and blood adverse effects. Sharquie regime was applied in treatment of early stages of mycosis fungoides and also demonstrated better results with much safer profile than other modalities of treatment. This mode of treatment is easy to be applied and could be used for longer time thus achieving long term remission and might prevent the progression of the disease.
5. Conclusions
- The present work had shown that methotrexate can be given safely as a first line therapy for patch and plaque stage mycosis fungoides in low split dose (7.5 mg every three days with excellent results and for longer time with no important clinical and laboratory complications. Thus it has good therapeutic and might prevent the progression of the disease.
Disclosure
- This study was an independent study and not funded by any drug companies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML