Khalifa E. Sharquie1, Adil A. Noaimi1, Nada Y. Noori2
1Department of Dermatology, College of Medicine, University of Baghdad, Iraqi and Arab Board for Dermatology and Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
2Department of Dermatology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Melasma is the major cause of facial melanosis where there are many topical therapies which end with high recovery rate but the relapse of disease is often also high. Imiquimod has been introduced as topical therapy of genital warts and skin malignancy where leukoderma and vitiligo were reported as side effects. Objectives: to evaluate imiquimod cream in low concentrations in treatment of melasma. Patients and Methods: This is an interventional, single-blinded, comparative outpatient study carried out in the Department of Dermatology, Baghdad Teaching Hospital during the period from July 2014 to October 2015. Thirty-four patients with melasma were included in this study. The patients were divided into three groups. Every patient was instructed to apply the treatment twice daily and to be seen every 2 weeks for 2 months and also instructed to apply a broad spectrum sunscreen with SPF >30 before sun exposure during treatment period. Results: Only twenty-four patients with melasma completed the study and all patients in group (A,B and C) were females. In group A, nine patients were treated with imiquimod cream 0.1612%. The percentage of improvement was (2.98%) and was statistically not significant (P value = 0.833229). In group B, eight patients were treated with imiquimod cream 0.3125%. There was a (3.49%) of improvement and was statistically not significant (P value = 0. 903421). In group C, seven patients treated with imiquimod cream 0.5882%. There was a (3.68%) of improvement and was statistically not significant (P value = 0.909153). The comparison of the percentage of improvement among the 3 groups showed no significant difference (P value = 0.966763). No side effects like leukoderma and vitiligo reported apart from slight burning sensation and irritation reported in few patients. Conclusions: Imiquimod cream in low concentrations (0.1612%, 0.3125% and 0.5882%) was useful as mild bleaching agent for treatment of melasma with no significant side effects. Further evaluation of this drug in treatment of other pigmentary diseases is highly needed to have more wide objective therapeutic results.
Keywords:
Imiquimod, Melasma, Treatment
Cite this paper: Khalifa E. Sharquie, Adil A. Noaimi, Nada Y. Noori, Imiquimod Cream in Low Concentrations as a New Topical Therapy for Melasma, American Journal of Dermatology and Venereology, Vol. 8 No. 4, 2019, pp. 66-72. doi: 10.5923/j.ajdv.20190804.03.
1. Introduction
Facial melanosis is a major cosmetic problem in Iraqi population and the commonest that have been recorded are melasma, lichen planus actinicus, frictional hypermelanosis, postinflammatory hyperpigmentation, acanthosis nigricans and phytophotodermatitis [1].Melasma is the commonest cause of facial melanosis where medical therapies often cause a high recovery rate but followed often by a high recurrence rate. There are many triggering factors for inducing melasma like sun light exposure, pregnancy, OCP, cosmetics and emotional stress [2].Imiquimod is an immune response modifier with the chemical structure(1-(2-methylpropyl)-1H-imidazo [4,5-c] quinolin-4-amine) [3] (trade name Aldara™) is a small molecule of the imidazoquinoline family, a group of nucleoside analogs [4].There are many mechanism of actions like topically applied imiquimod induces local cytokine production from keratinocytes and other cells [5,6]. Imiquimod has been shown to interact with Toll-like receptor 7 and (to a lesser degree) 8 [7] resulting in the secretion of proinflammatory cytokines, such as IFN-α and IFN-γ, tumor necrosis factor-α, IL-1, IL-6, IL-10, IL-12, granulocyte colony-stimulating factor, granulocyte-monocyte colony-stimulating factor, and the chemokines IL-8, macrophage inflammatory protein 1α, and monocyte chemotactic protein 1 [8,9]. Imiquimod also stimulates the innate immune response by increasing natural killer cell activity, activating macrophages and Langerhans cells, and inducing proliferation and maturation of B lymphocytes. Additionally, imiquimod has direct proapoptotic activity on tumor cells [10].Imiquimod has demonstrated antiangiogenic properties in vitro and in a series of case reports [10]. The antiangiogenic properties of imiquimod stem from the production of antiangiogenic cytokines including IFN-γ, IL-10, and IL-12; the upregulation of endogenous antiangiogenic mediators, including tissue inhibitor of matrix metalloproteinase; and downregulation of the proangiogenic factors, basic fibroblast growth factor, and matrix metalloproteinase 9 [11].Imiquimod has been introduced as effective topical therapy for treatment of genital wart and skin cancers [12,13] but unfortunately, there are number of published reports on vitiligo-like hypopigmentation have been recorded as adverse side effects [14].The aim of present work is to use imiquimod cream in low concentrations in treatment of melasma and to record any side effects especially vitiligo.
2. Patients and Methods
This is an interventional, single-blinded, comparative outpatient study carried out in the Department of Dermatology, Baghdad Teaching Hospital during the period from July 2014 to October 2015. The nature and aim of this study were explained for each patient. Consent was taken from them before starting the therapy, after full explanation about the nature of the disease, course, the procedure of treatment,prognosis and the need for pre and post treatment photographs. Also, the ethical approval was given by the Scientific Council of Dermatology and Venereology-Iraqi Board for Medical Specializations.Inclusion Criteria: ▪ Patients clinically presented with different types of melasma were included.▪ Patients using therapy must stop any treatment for 2 months prior to the study.Exclusion Criteria: ▪ Pregnant and lactating females.▪ Patients with chronic illness like :liver, kidney, heart, blood dyscrasia, connective tissue diseases and any endocrine disease that interfere with skin pigmentation.▪ Patients receiving drugs that interfere with skin pigmentation especially oral contraceptive pills or any drug that cause pigmentation to the skin.▪ Immune suppressed patients. Full history was taken from each patient stressing on the: age, duration of melasma, marital status and if the patient married ask about the number of pregnancy and if the melasma occur during pregnancy or not, family history, sun exposure (outdoor or indoor), use of cosmetics, smoking, drug history and hair removal by which method and its frequency, also asked about premenstrual flare up and use of oral contraceptive pills. The diagnosis was made on clinical bases and Wood’s light examination.Thirty four females patients with melasma were included in the study.A careful examination of melasma was done at base line and in follow up visits including the following: * Morphology of melasma: butterfly, mask shape and localized. * Wood’s light examination for all patients to assess the depth of pigmentation and response of therapy.* Photographs for all patients were performed by using the camera of Ipad air 1 with 5 megapixels in the same place and distance with fixed illumination. * Calculation of melasma area and severity index (MASI) score was carried out for each patient.Melasma Area and Severity Index (MASI) score:In this system the face is divided into 4 areas. Forehead, right malar, left malar and chin. Figure (1)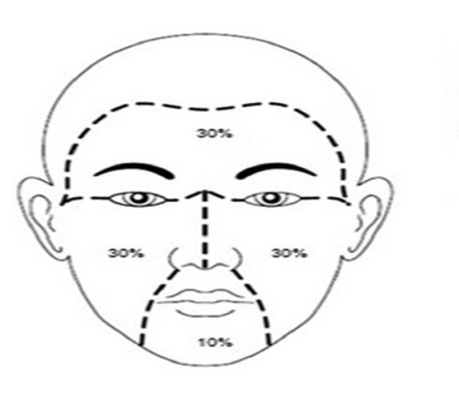 | Figure (1). MASI score divided the face into 4 areas (30% for forehead, 30% for right malar, 30% for left malar and 10% for chin) |
The melasma in each of these areas was graded on three variables: 1- Percentage of the total area involved on a scale: this was measured by using transparent square paper. By this method the melasma and the total surface areas were measured accurately by square centimeters, then the percentage of the melasma area relative to the total area of the same region was measured and scoring was done as follows:1 = less than 10%2 = 10-29%3 = 30-49%4 = 50-69%5 = 70-89%6 = 90-100%2- Darkness: scoring from 0-4 was assessed according to 4 special colour charts. 3- Homogeneity: scoring from 0-4.Scale 1 = Specks of involvementScale 2 = Small patchy areas of involvement < 1.5 cm in diameterScale 3 = Patches of involvement > 2cm in diameterScale 4 = Uniform skin involvement without any clear areasThe MASI score was calculated by the following equation: Total MASI score = 0.3(DF+HF)AF + 0.3(DMR+HMR) AMR + 0.3(DML+HML) AML + 0.1(DC+HC) AC.Where D is darkness, H is homogeneity, A is area, F is forehead, MR is right malar, ML is left malar and C is chin. The maximum MASI score is 48.The patients were divided into three groups: group A, B and C.The drug used in this study was imiquimod cream 5% (AldaraTM) (the sachet contain 250 mg of cream) and the company of the drug was (MEDA).Group AThirteen patients with melasma were included in this part of the study.Preparations and treatment plan:One sachet of imiquimod cream 5% was mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,1612% and put in closed container. The aqua rosa cream kept in a refrigerator before mixing in summer while in winter kept in room temperature. This amount was for 2 weeks. The patients were instructed to apply the cream 2 times per day over the area of melasma daily for 2 months by fingertip method with gentle message.Group BThirteen patients with melasma were included in this part of the study.Preparation and treatment plan:Two sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have the final concentration was 0,3125% and put in closed container. This amount was for 2 weeks. The patients were instructed to apply the cream by the same manner in group A.Group CEight patients with melasma were included in this part of the study.Preparation and treatment plan:Four sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,5882% and put in closed container. This amount was for 2 weeks.The instructions to the patients to apply the cream were the same in group A.All patients in group A, B and C were instructed to avoid sun exposure as much as possible during and after treatment, and to use sun screen with SPF>30 during day light and repeated every 3 hours. The patients were seen regularly every 2 weeks for 2 months to assess the response to treatment and taking photos for each patient and recording the side effects if present.Data were statistically described in terms of range, mean, standard deviation (±SD), frequencies (number of cases) and relative frequencies (percentages). Comparison between first visit and last visit at the same group was done by using paired t test while comparison between groups about the percentage of improvement was done by using independent t test. All statistical calculations were done by using Epi info 6 statistical programs.
3- Homogeneity: scoring from 0-4.Scale 1 = Specks of involvementScale 2 = Small patchy areas of involvement < 1.5 cm in diameterScale 3 = Patches of involvement > 2cm in diameterScale 4 = Uniform skin involvement without any clear areasThe MASI score was calculated by the following equation: Total MASI score = 0.3(DF+HF)AF + 0.3(DMR+HMR) AMR + 0.3(DML+HML) AML + 0.1(DC+HC) AC.Where D is darkness, H is homogeneity, A is area, F is forehead, MR is right malar, ML is left malar and C is chin. The maximum MASI score is 48.The patients were divided into three groups: group A, B and C.The drug used in this study was imiquimod cream 5% (AldaraTM) (the sachet contain 250 mg of cream) and the company of the drug was (MEDA).Group AThirteen patients with melasma were included in this part of the study.Preparations and treatment plan:One sachet of imiquimod cream 5% was mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,1612% and put in closed container. The aqua rosa cream kept in a refrigerator before mixing in summer while in winter kept in room temperature. This amount was for 2 weeks. The patients were instructed to apply the cream 2 times per day over the area of melasma daily for 2 months by fingertip method with gentle message.Group BThirteen patients with melasma were included in this part of the study.Preparation and treatment plan:Two sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have the final concentration was 0,3125% and put in closed container. This amount was for 2 weeks. The patients were instructed to apply the cream by the same manner in group A.Group CEight patients with melasma were included in this part of the study.Preparation and treatment plan:Four sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,5882% and put in closed container. This amount was for 2 weeks.The instructions to the patients to apply the cream were the same in group A.All patients in group A, B and C were instructed to avoid sun exposure as much as possible during and after treatment, and to use sun screen with SPF>30 during day light and repeated every 3 hours. The patients were seen regularly every 2 weeks for 2 months to assess the response to treatment and taking photos for each patient and recording the side effects if present.Data were statistically described in terms of range, mean, standard deviation (±SD), frequencies (number of cases) and relative frequencies (percentages). Comparison between first visit and last visit at the same group was done by using paired t test while comparison between groups about the percentage of improvement was done by using independent t test. All statistical calculations were done by using Epi info 6 statistical programs.
3. Results
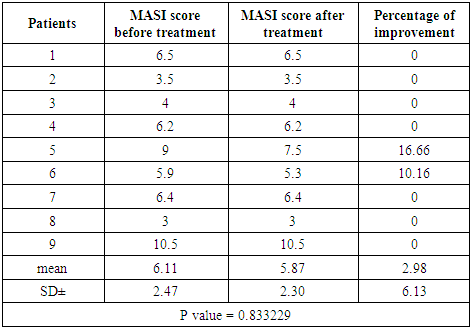
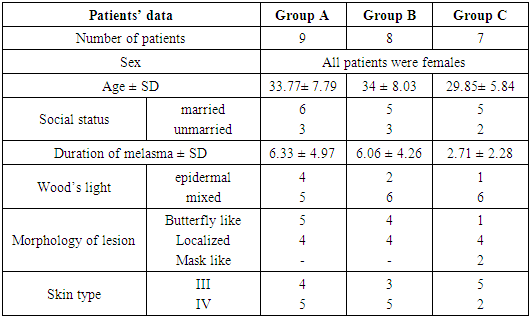
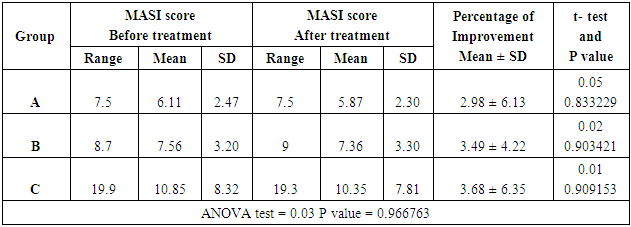
Thirty four females patients with melasma were included in this study. Family history of melasma was positive in 21 patients (61.76%). Ten patients were defaulted from the study for unknown reasons.Group A (Imiquimod cream 0.1612%)Patients’ data:Thirteen patients with melasma were included in this study. Four patients were defaulted, nine patients completed the study, their ages ranged from 23 to 44 years with a mean of 33.77 ± SD of 7.79 years.Six patients (66.66%) were married and 3 patients (33.33%) were unmarried. Three patients from 6 married had history of melasma during pregnancy.Family history of melasma was positive in 7 patients.The duration of melasma ranged from 1 to 16 years with a mean ± SD of 6.33 ± 4.97 years.According to Fitzpatrick’s classification 4 patients were skin type III and 5 patients with skin type IV. Wood’s light examination showed epidermal type in 4 patients and 5 patients had mixed type.Morphological forms of melasma were butterfly like in 5 patients (55.55%) and localized in 4 patients (44.44%). Clinical results:The MASI score before treatment was 6.11 ± 2.47 while after treatment MASI score changed into 5.87 ± 2.30, so the average decrease in MASI score was 0.24.This represents a 2.98% of improvement and was statistically not significant (P value = 0.833229). Table (1)Side effects:There were no any side effects recorded from any patient.Table (1). MASI score before and after 2 months of treatment for group A
 |
| |
|
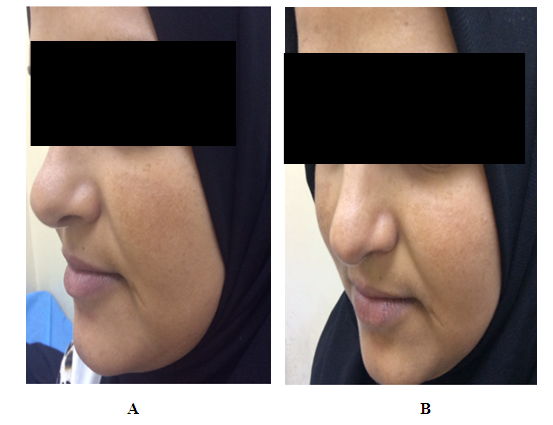 | Figure (2). A twenty three years old female. A. Before therapy MASI score was 9. B. After two months of therapy with imiquimod cream 0.1612%, MASI score became 7.5 |
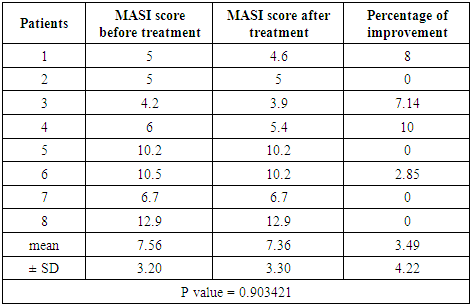
Group B (Imiquimod cream 0.3125%)Patients’ data:Thirteen patients with melasma were included in this study. Five patients were defaulted, eight patients completed the study, their ages ranged from 20 to 45 years with a mean of 34 ± SD of 8.03 years.Five patients (62.5%) were married and 3 patients (37.5%) were unmarried. Three patients from 5 married had history of melasma during pregnancy.Family history of melasma was positive in all patients.The duration of melasma ranged from 1.5 to 15 years with a mean ± SD of 6.06 ± 4.26 years. According to Fitzpatrick’s classification 3 patients were skin type III and 5 patients with skin type IV. Wood’s light examination showed epidermal type in 2 patients and 6 patients had mixed type. Morphological forms of melasma were butterfly like in 4 patients (50%) and localized in 4 patients (50%).Clinical results:The MASI score before treatment was 7.56 ± 3.20 while after treatment MASI score changed into 7.36 ± 3.30, so the average decrease in MASI score was 0.2.This represents a 3.49% of improvement and was statistically not significant (P value = 0.903421) Table (2).Table (2). MASI score before and after 2 months treatment for group B
 |
| |
|
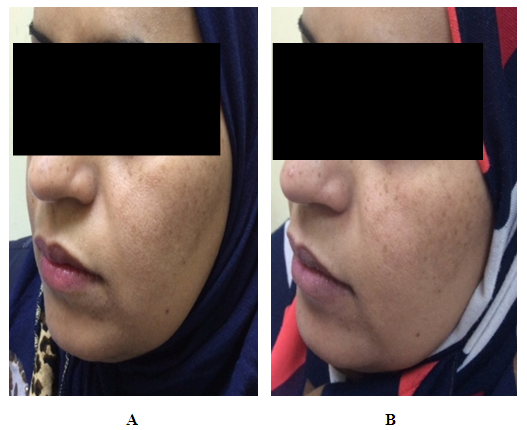 | Figure (3). A thirty seven years old female. A. Before therapy MASI score was 4.2. B. After two months of therapy with imiquimod cream 0.3125%, MASI score became 3.9 |
Side effects:During the treatment no side effects were recorded a part from slight burning sensation(this burning sensation continued shortly after application then disappeared) which was occurred in two patients and the same patient had also mild erythema in the normal skin adjacent to area of melasma appeared in the end of treatment period.Group C (Imiquimod cream 0.5882%)Patients’ data:Eight patients with melasma were included in this study. One patient was defaulted, seven patients completed the study, their ages ranged from 21 to 37 years with a mean of 29.85 ± SD of 5.84 years.Five patients (71.42%) were married and 2 (28.57%) were unmarried. Two patients from 5 married had history of melasma during pregnancy.Family history of melasma was positive in 3 patients.The duration of melasma ranged from 1 to 6 years with a mean ± SD of 2.71 ± 2.28 years. According to Fitzpatrick’s classification 5 patients were skin type III and 2 patients with skin type IV. Wood’s light examination showed epidermal type 1patient and 6 patients had mixed type.Morphological forms of melasma were mask like in 2 patients (28.57%), butterfly like in 1 patient (14.28%) and localized in 4 patients (57.14%). 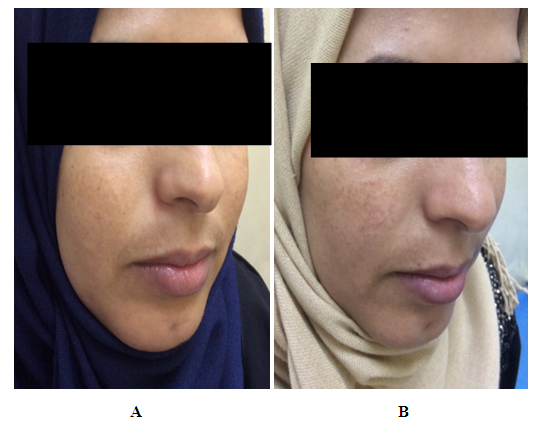 | Figure (4). A twenty seven years old female. A. Before therapy MASI score was 6.2. B. After two months of therapy with imiquimod cream 0.5882%, MASI score became 5.3 |
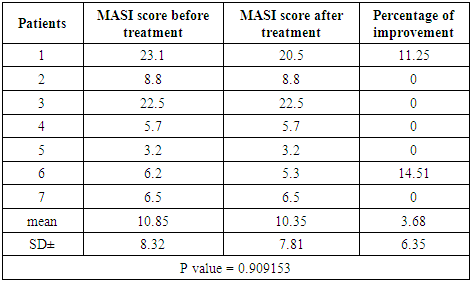
Clinical results:The MASI score before treatment was 10.85 ± 8.32 while after treatment MASI score changed into 10.35 ± 7.81, so the average decrease in MASI score was 0.5. This represents a 3.68% of improvement and was statistically not significant (P value = 0.909153). Table (3)Table (3). MASI score before and after 2 months treatment for group C
 |
| |
|
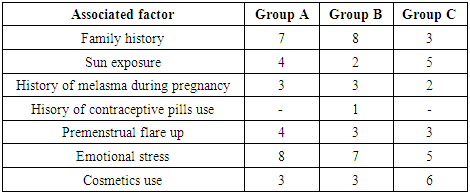
Side effects:During treatment course, two patients complained from slight burning sensation and two patients complained from irritation in the same area of melasma.The comparison of the percentage of improvement among the 3 groups showed no significant difference (P value = 0.966763). Table (6) Table (4). Patients’ data in three groups
 |
| |
|
Table (5). Associated factors with melasma in three groups
 |
| |
|
Table (6). Comparison among patients in three groups by using ANOVA test
 |
| |
|
4. Discussion
Melasma is the most common cause of facial melanosis [15] which is associated with considerable psychological impacts [16].In addition to avoidance of aggravating factors like oral pills and ultraviolet exposure, topical therapy has remained the mainstay of treatment. Multiple options for topical treatment are available; of which hydroquinone is the most commonly prescribed agent. In addition other topical agents for which varying degrees of evidence for clinical efficacy exist include azelaic acid, kojic acid, retinoid, topical steroids, glycolic acid, arbutin[16], topical zinc sulphate [17,18] and lactic acid [19]. Topical medications modify various stages of melanogenesis, the most common mode of action being inhibition of the enzyme, tyrosinase. Combination therapy is the preferred mode of treatment for the synergism and reduction of untoward effects. The most popular combination consists of hydroquinone, a topical steroid, and retinoic acid [16].Dermabrasion has been tried as curable therapy for melasma where five hundred and thirty-three patients with melasma were treated by mechanical dermabrasion using a rotatory diamond fraise. Four hundred and ten patients were available for long-term follow-up (mean follow-up time 5 years, range 1-9 years). Out of 410 patients, 398 (97%) achieved persistent clearance of melasma; in the remaining cases, there was partial recurrence after initial clearance [20]. But the medical therapy remains the first line aiming to achieve high recovery with low recurrence rate. Imiquimod 5% cream, as an immune response modifier and a safe drug, is used to treat condylomata acuminata, basal cell carcinoma, Bowen’s disease, common and planter warts, molluscum contagiosum, and other disorders. However, mild - to - moderate, local and systemic, adverse effects of imiquimod may occasionally occur. Among its adverse effects imiquimod – induced vitiligo which should be anticipated when dermatologists prescribe this drug [3].Imiquimod causes hypopigmentation through the following mechanisms: it increases production of proinflammatory cytokines, mainly interferon (IFN)-α, tumor necrosis factor (TNF)-α, and IL-6, IL-8, IL-10, IL-12, all of which augment the type 1 helper T-cell (TH1) response which is found to be prominent in the pathogenesis of vitiligo [21]. Also imiquimod enhances antigen presentation by stimulating CD8+ T-cell activation and inducing Langerhans cell maturation. Vitiligo-like depigmentation may result from the destruction of melanocytes by CD8+ T-cells directed to melanocyte surface antigens after antigen presentation is enhanced by imiquimod [22]. Also recently it was reported that human melanocytes express toll like receptor 7 (TLR7). When applied topically, imiquimod binds to TLR7 followed by stimulation of various cytokines which induce the above mentioned T lymphocytic response [23]. It has been found that the some of the same cytokines induced by imiquimod, IL-6, IL-1, and TNF-α, are paracrine inhibitors of melanocytes and exhibit significantly higher expression in vitiligo skin compared to healthy skin. All three cytokines were found to elicit a dose-dependent decrease in the activity of the enzyme tyrosinase [24]. Imiquimod also has a direct action on melanocytes via apoptosis of melanocytes. This action is related to reduction of expression of Bcl-2 and/or an increase in the proapoptotic stimulus (cytotoxic T lymphocytes, natural cytotoxic T cells/killer cells, granzymes B, Fas, TNF, Bax) [25]. Also, depigmentation did not extend to areas that had not been treated with imiquimod [22].Therefore, it is possible that imiquimod may cause damage of melanocytes by direct influence on cells as well as inducing acquired immunity indirectly, thus inducing vitiligo like lesions.The aim of present work to test imiquimod efficacy in treatment of melasma and to record any side effects as a sequelae of therapy.The results of this study, where twenty four patients with melasma with twice daily application for 2 months of imiquimod in 3 concentrations (0.1612%, 0.3125% and 0,5882%) showed a mild percentage of improvement (2.98%, 3.49% and 3.68% respectively) and there was no statistically significant difference between each group before and after treatment and between different groups.Also the results showed that imiquimod induced no important adverse effects like leukoderma and vitiligo although this drug had been used twice a day for two months. While other studies that reported vitiligo as a side effect, patients had applied imiquimod cream 5% three times a week for treatment of genital warts.
5. Conclusions
Imiquimod cream in low concentrations (0.1612%, 0.3125% and 0.5882%) was useful as mild bleaching agent for treatment of melasma with no significant side effects. Further evaluation of this drug in treatment of other pigmentary diseases is highly needed to have more wide objective therapeutic results.
References
| [1] | Sharquie KE, Noaimi AA. Gazelle eye like facial melanosis (clinico-histopathological study). Pigmentary Disorders. 2014; 1(2): 1-4. |
| [2] | Sonthalia S. Etiopathogenesis of Melasma. In: Sarkar R. Melasma: A Monograph. 1st ed. India: Jaypee; 2015. p.6-14. |
| [3] | Li W, Xin H, Ge L, Song H, Cao W. Induction of vitiligo after imiquimod treatment of condylomata acuminata. BMC Infect Dis. 2014; 14:329. |
| [4] | Ryu J and Yang F. A review of topical imiquimod in the management of basal cell carcinoma, actinic keratoses, and other skin lesions. Clinical Medicine: Therapeutics. 2009;1: 1557-1575. |
| [5] | Chang YC, Madkan V, Cook-Norris R, Sra K, Tyring S. Current and potential uses of imiquimod. South Med J. 2005; 98(9): 914-20. |
| [6] | Sapijaszko MJ. Imiquimod 5% cream (Aldara) in the treatment of basal cell carcinoma. Skin Therapy Lett. 2005; 10(6): 2-5. |
| [7] | Kirnbauer R, Lenz P. Human Papillomaviruses. In:Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rded. China: Elsevier Saunders; 2012. p.1316. |
| [8] | Schiller M, Metze D, Luger TA, Grabbe S, Gunzer M. Immune response modifiers--mode of action. Exp Dermatol. 2006; 15(5): 331-41. |
| [9] | Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006; 126(6): 1338-47. |
| [10] | Esparza EM, Sidbury R. Topical Immunomodulators. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. Fitzpatrick’s Dermatology in General Medicine. 8th ed. McGrawHill; 2012.p.2692-2696. |
| [11] | Li VW, Li WW, Talcott KE, Zahi AW. Imiquimod as an antiangiogenic agent. J Drugs Dermatol. 2005; 4(6):708-17. |
| [12] | Edwards L, Ferenczy A, Eron L, Baker D, Owens ML, Fox TL, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human Papilloma Virus. Arch Dermatol. 1998; 134(1): 25-30. |
| [13] | Marks R, Gebauer K, Shumack S, Amies M, Bryden J, Fox TL, et al. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose-response trial. J Am Acad Dermatol. 2001; 44(5): 807-13. |
| [14] | Brown T, Zirvi M, Cotsarelis G, Gelfand JM. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005; 52(4): 715-6. |
| [15] | Kubba A, Batrani M. Histopathological evaluation of melasma and other facial hyperchromias. In: Lahiri K, Chatterjee M, Sarkar R. PIGMENTARY DISODERS A Comprehensive Compendium, 1st ed. India: Jaypee; 2014. p.288. |
| [16] | Bandyopadhyay D. Topical treatment of melasma. Indian J Dermatol. 2009; 54(4): 303-309. |
| [17] | Salman HA. Topical 10% zinc sulphate for treatment of melasma. A comparative clinical trial of 3 formulations solution, ointment and cream. A Thesis Submitted to the Scientific Council of Dermatology and Venereology as a Partial Fulfillment for the Degree of Fellowship of Iraqi Board for Medical Specializations in Dermatology and Venereology. 2006. |
| [18] | Sharquie KE, Al-Mashhadani SA, Salman HA. Topical 10% zinc sulfate solution for treatment of melasma.Dermatol Surg. 2008; 34(10): 1346-9. |
| [19] | Sharquie KE, Noaimi AA, Al-Hemiari ON. Lactic acid cream 6% versus 10% zinc sulfate cream as comparative study in treatment of melasma. Journal of Dental and Medical Sciences. 2015; 14(6): 105-115. |
| [20] | Kunachak S, Leelaudomlipi P, Wongwaisayawan S. Dermabrasion: a curative treatment for melasma. Aesthetic Plast Surg. 2011; 25(2):114-7. |
| [21] | Alghamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol. 2011; 25(7): 749-57. |
| [22] | Senel E, Seckin D. Imiquimod- induced vitiligo-like depigmentation. Indian J Dermatol Venerol Leprol. 2007; 73(6): 423. |
| [23] | Kang HY, Park TJ, Jin SH. Imiquiomd, a toll-like receptor 7 agonist, inhibits melanogenesis and proliferation of human melanocytes. J Invest Dermatol. 2009; 129(1): 243-6. |
| [24] | Al-Dujaili Z, Hsu S. Imiquimod- induced vitiligo. Dermatol Online J. 2007; 13(2):10. |
| [25] | Kim CH, Ahn JH, Kang SU, Hwang HS, Lee MH, Pyun JH, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010; 302(4): 301-6. |



 3- Homogeneity: scoring from 0-4.Scale 1 = Specks of involvementScale 2 = Small patchy areas of involvement < 1.5 cm in diameterScale 3 = Patches of involvement > 2cm in diameterScale 4 = Uniform skin involvement without any clear areasThe MASI score was calculated by the following equation: Total MASI score = 0.3(DF+HF)AF + 0.3(DMR+HMR) AMR + 0.3(DML+HML) AML + 0.1(DC+HC) AC.Where D is darkness, H is homogeneity, A is area, F is forehead, MR is right malar, ML is left malar and C is chin. The maximum MASI score is 48.The patients were divided into three groups: group A, B and C.The drug used in this study was imiquimod cream 5% (AldaraTM) (the sachet contain 250 mg of cream) and the company of the drug was (MEDA).Group AThirteen patients with melasma were included in this part of the study.Preparations and treatment plan:One sachet of imiquimod cream 5% was mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,1612% and put in closed container. The aqua rosa cream kept in a refrigerator before mixing in summer while in winter kept in room temperature. This amount was for 2 weeks. The patients were instructed to apply the cream 2 times per day over the area of melasma daily for 2 months by fingertip method with gentle message.Group BThirteen patients with melasma were included in this part of the study.Preparation and treatment plan:Two sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have the final concentration was 0,3125% and put in closed container. This amount was for 2 weeks. The patients were instructed to apply the cream by the same manner in group A.Group CEight patients with melasma were included in this part of the study.Preparation and treatment plan:Four sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,5882% and put in closed container. This amount was for 2 weeks.The instructions to the patients to apply the cream were the same in group A.All patients in group A, B and C were instructed to avoid sun exposure as much as possible during and after treatment, and to use sun screen with SPF>30 during day light and repeated every 3 hours. The patients were seen regularly every 2 weeks for 2 months to assess the response to treatment and taking photos for each patient and recording the side effects if present.Data were statistically described in terms of range, mean, standard deviation (±SD), frequencies (number of cases) and relative frequencies (percentages). Comparison between first visit and last visit at the same group was done by using paired t test while comparison between groups about the percentage of improvement was done by using independent t test. All statistical calculations were done by using Epi info 6 statistical programs.
3- Homogeneity: scoring from 0-4.Scale 1 = Specks of involvementScale 2 = Small patchy areas of involvement < 1.5 cm in diameterScale 3 = Patches of involvement > 2cm in diameterScale 4 = Uniform skin involvement without any clear areasThe MASI score was calculated by the following equation: Total MASI score = 0.3(DF+HF)AF + 0.3(DMR+HMR) AMR + 0.3(DML+HML) AML + 0.1(DC+HC) AC.Where D is darkness, H is homogeneity, A is area, F is forehead, MR is right malar, ML is left malar and C is chin. The maximum MASI score is 48.The patients were divided into three groups: group A, B and C.The drug used in this study was imiquimod cream 5% (AldaraTM) (the sachet contain 250 mg of cream) and the company of the drug was (MEDA).Group AThirteen patients with melasma were included in this part of the study.Preparations and treatment plan:One sachet of imiquimod cream 5% was mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,1612% and put in closed container. The aqua rosa cream kept in a refrigerator before mixing in summer while in winter kept in room temperature. This amount was for 2 weeks. The patients were instructed to apply the cream 2 times per day over the area of melasma daily for 2 months by fingertip method with gentle message.Group BThirteen patients with melasma were included in this part of the study.Preparation and treatment plan:Two sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have the final concentration was 0,3125% and put in closed container. This amount was for 2 weeks. The patients were instructed to apply the cream by the same manner in group A.Group CEight patients with melasma were included in this part of the study.Preparation and treatment plan:Four sachets of imiquimod cream 5% were mixed gently with 7.5 gm of aqua rosa cream to have final concentration was 0,5882% and put in closed container. This amount was for 2 weeks.The instructions to the patients to apply the cream were the same in group A.All patients in group A, B and C were instructed to avoid sun exposure as much as possible during and after treatment, and to use sun screen with SPF>30 during day light and repeated every 3 hours. The patients were seen regularly every 2 weeks for 2 months to assess the response to treatment and taking photos for each patient and recording the side effects if present.Data were statistically described in terms of range, mean, standard deviation (±SD), frequencies (number of cases) and relative frequencies (percentages). Comparison between first visit and last visit at the same group was done by using paired t test while comparison between groups about the percentage of improvement was done by using independent t test. All statistical calculations were done by using Epi info 6 statistical programs. 


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




