-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2019; 8(3): 49-54
doi:10.5923/j.ajdv.20190803.04

Lupus Miliaris Disseminatus Faciei and Granulomatous Rosacea are Twin of One Disease: Named 'Rosoid Facial Granulomatosis
Khalifa E. Sharquie1, Wesal K. Al-Janabi2, Fatema A. Al-Jaralla3
1Department of Dermatology, College of Medicine, University of Baghdad, Iraqi and Arab Board for Dermatology & Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
2Dermatology Center, Medical City, Baghdad, Iraq
3Departments of Dermatology, College of Medicine, Baghdad University, Baghdad, Iraq
Correspondence to: Wesal K. Al-Janabi, Dermatology Center, Medical City, Baghdad, Iraq.
| Email: | 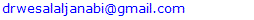 |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Granulomatous rosacea (GR) is considered by some authors as a variant of rosacea, GR can often be difficult to differentiate from other facial granulomatous dermatoses, it can mimic lupus miliaris disseminatus faciei (LMDF), micropapular sarcoidosis, and tuberculosis. Of these, its relationship with LMDF is most intriguing. There is much debate about the etiopathogenesis, the clinical and histopathological features of LMDF and GR, mostly because of the overlapping features between these two conditions.This study evaluate the clinical and histopathological features of LMDF and GRand compare these features with what had been published in medical literature aiming to find one representative entity. Thirty patients with GR and LMDF were collected, 23 cases with LMDF and seven cases with GR. The mean age of patients in both diseases was comparable (P-value 0.191); with GR was 41 (range 26-60) with SD=11.7 years, and 48.47 (range 25-70) with SD= 14.89 years in patients with LMDF. The GR cases comprised five males and two female patients with male: female ratio 2.5:1, whereas LMDF cases were 9 males and 14 female patients with male: female ratio 1: 1.5. All the studied cases showed facial involvement mainly, with common periorbital involvement in LMDF. There was additional extra facial involvement in three (13.4%) cases of LMDF, versus none in GR patients. In LMDF, the upper and lower eyelids were involved in 10(43.4%) cases, while involvement of the upper eyelids only occurred in four (17.3%) cases of LMDF, whereas no eyelid lesions seen in GR. The rash in GR was small mostly skin colored papules without pustules, versus large erythematous to dusky red nodules and sometimes pustules in five (21.7%) cases of LMDF. No fixed erythema or phymatous changes seen in both diseases. In both GR and LMDF, Histopathological examination showed a granulomatous inflammatory reaction around and sometimes embedding the hair follicles probably because both conditions affecting the face where there are plenty of hair follicles. In GR, epithelioid granuloma without noticeable necrosis, whereas LMDF showed a well-formed granuloma and more severe inflammatory reaction with dominating neutrophils inside the hair follicles forming abscesses and obvious necrosis. However, Giant cells were detected in both conditions. Thus, after reviewing literature and the results of the present study, it seems that there are many common clinical and histopathological features between GR and LMDF. Accordingly, we can conclude that both conditions represent one entity and manifest as twin of the same disease and probably not related to ordinary rosacea. Hence, we propose the term “rosoid facial granulomatosis” as an umbrella to include both conditions at the two ends of one spectrum.
Keywords: Granulomatous Rosacea, Lupus Milliaris Dissiminatus Faciei, Rosoid facial Granulomatosis, Iraq
Cite this paper: Khalifa E. Sharquie, Wesal K. Al-Janabi, Fatema A. Al-Jaralla, Lupus Miliaris Disseminatus Faciei and Granulomatous Rosacea are Twin of One Disease: Named 'Rosoid Facial Granulomatosis, American Journal of Dermatology and Venereology, Vol. 8 No. 3, 2019, pp. 49-54. doi: 10.5923/j.ajdv.20190803.04.
Article Outline
1. Introduction
- Granulomatous rosacea is characterized by hard, yellow, brown, or red cutaneous papules or nodules with normal appearing background, however, they may be severe and lead to scarring. They can vary in size among patients but are monomorphic in each individual patient, and typically appear on the cheeks and periorificial areas. Granulomatous rosacea may occur in locations other than those with phymas. The presence of other rosacea signs is not necessary for a diagnosis of the granulomatous rosacea variant. [1-3]Histological examination may reveal a spectrum of findings depending on the subtype of disease. These findings may range from a lymphohistiocytic response to a predominantly histiocytic response along with formation of noncaseating epithelioid-cell granulomas. These granulomas are of unknown etiology and may resemble those present in sarcoidosis. [4] A prominent characteristic of granulomatous rosacea is the presence of epithelioid histiocytes and multinucleate giant cells in tuberculoid granulomata, which may be centred on ruptured hair follicles. Nonpustular lesions show a nonspecific perivascular and perifollicular lymph histiocytic infiltrate accompanied by occasional multinucleated cells, plasma cells, neutrophils, and eosinophils. Papulopustular lesions show pronounced granulomatous inflammation but occasional perifollicular abscesses. [3,5,6]Granulomatous rosacea was reported primarily in middle- aged women and in association with immunosuppression. [7,8]Lupus miliaris disseminatus faciei (LMDF) is another uncommon granulomatous inflammatory skin disease of unknown etiology affecting the face. It was first described by Tilbury Fox et al in 1878. [9] It is characterized by multiple discrete yellow-brown to red, dome shaped papules on the medial and lateral areas of the face, which often extends to the neck and chin. LMDF often involves the eyelids, especially the lower eyelids. [10] However, an increasing number of cases were reported with extra facial involvement including the axillae, neck, scalp, legs, trunk and genitalia. [11-15] Patients with exclusively extra facial lesions (neck and chest) have been described. [16] It is usually seen in adults between the second and the fourth decades of life, [17] although it may develop also in children [18,19] and in elderly patients. [18,20] Most published reports reviewed and cited in the Bibliography describe solely or predominantly male patients. The lesions usually regress spontaneously within 12 to 24 months leaving depressed scars. [21]The histopathology of LMDF is characterized by a dermal granulomatous infiltrate. The changes vary according to the age of lesions (early, fully developed, and late stage). Early stage lesions comprise a perivascular and peri adnexal lympho-histiocytic infiltrate. In the fully developed stage, the following spectrum of changes can be seen: epithelioid cell granuloma with central necrosis; Epithelioid cell granuloma without central necrosis (sarcoidal type granuloma); Epithelioid cell granuloma with abscess; In addition, non- granulomatous, non-specific inflammatory infiltration. Late lesions show extensive perifollicular fibrosis with nonspecific cell infiltration. [21-23]Still the pathogenesis of the LMDF is not known. [21] It was considered before to be a tuberculid, many authors now believe that it is an extreme variant of granulomatous rosacea. Moreover, others have the opinion that it is a distinct entity. [19]. However, recently neither GR nor LMDF were related to a tuberculous aetiology. [24]The objective of the present work is to report cases of GR and LMDF and to compare their features with what had been described in literature aiming to reach a conclusion regarding their relation to rosacea and whether they are representing one entity apart from rosacea.
2. Patients and Methods
- This study was carried out in the Dermatology Centre, Baghdad Medical City, Baghdad, Iraq during the period from July 2013 to March 2019. All the cases of GR and LMDF, over the past 6 years were collected and evaluated for the clinical features of both diseases. Histopathological assessment was carried out in some cases to confirm the clinical diagnosis.
3. Results
3.1. Clinical Study
- A total of 30 patients, 23 cases of LMDF and 7 cases of GR and were analysed. None of the GR or LMDF cases had any systemic clinical symptoms suggestive of TB or sarcoidosis. The clinical and pathological features are summarized in Table 1.
|
3.2. Histopathological Findings
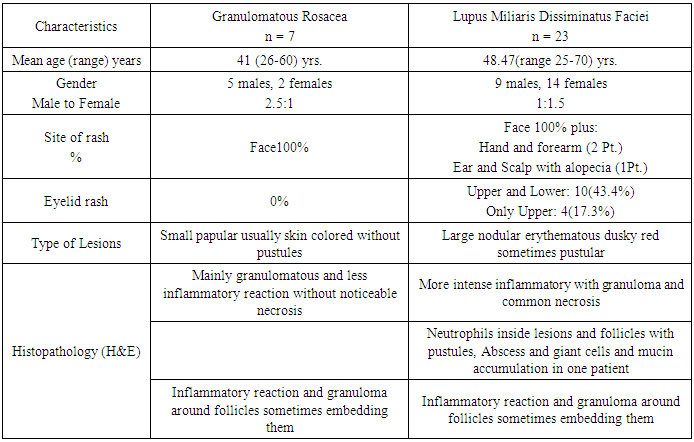
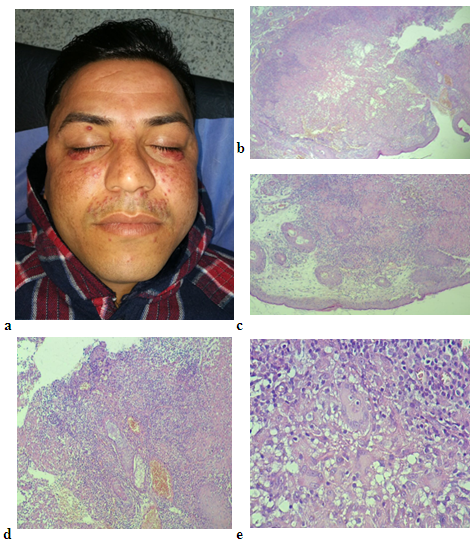
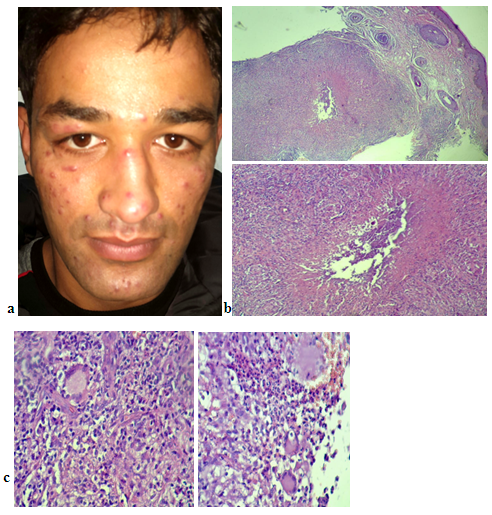
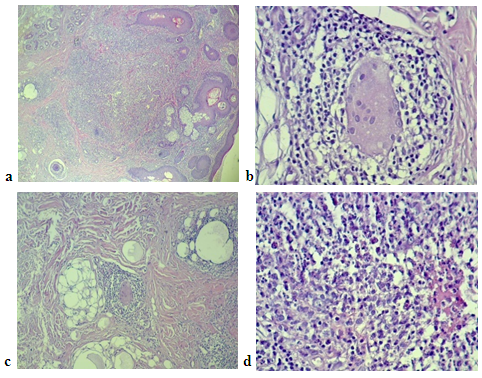
- Histological examination of slides stained with hematoxylin and eosin showed both GR and LMDF to have granulomatous inflammatory reaction around and sometimes embedding the hair follicles probably because both conditions affecting the face, where it is rich in hair follicles. In granulomatous rosacea epithelioid granuloma, (Fig. 1). With little necrosis with mild inflammatory infiltrate were seen, while LMDF showed more intense inflammatory reaction with granuloma formation, necrosis and neutrophils inside the follicles with pustules and abscess formation, (Fig. 2, 3), but one case without necrosis, (Fig.7). Giant cells were seen in both conditions. One patient with LMDF had mucin accumulation within the granuloma.
 | Figure 1 (a, b). (a) a female patient with granulomatous rosacea (b) H&E stain × 40 showing a well-formed granuloma without necrosis |
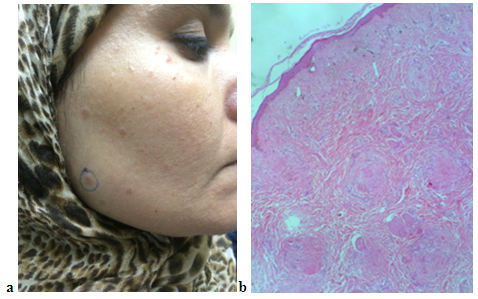
 | Figure 4 (a, b). (a) Forty-seven years female patient with LMDF (b) extra facial lesions including forearm and hands |
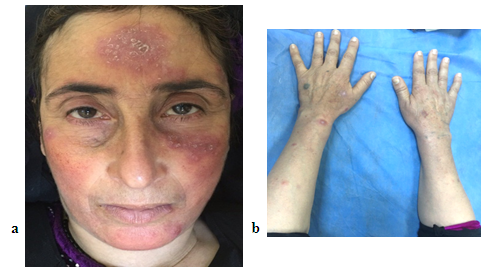
 | Figure 5. The 66 years LMDF female patient with facial and extra facial lesions including chest and forearm |
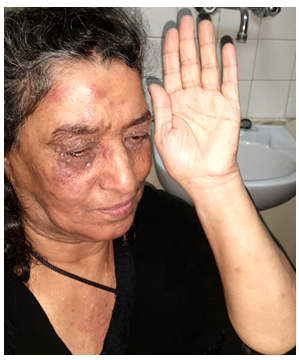
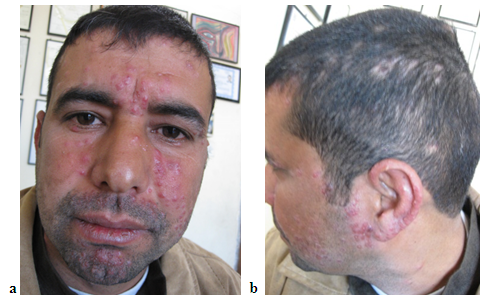 | Figure 6 (a, b). (a) and (b) thirty-four years male patient with LMDF having facial and extra facial lesions including ear and scalp lesions with alopecia |
4. Discussion
- Rosacea is a chronic inflammatory skin disorder mostly affecting light-skinned middle-aged people, seen up to 10% of the population and mostly in females. [25] Rosacea diagnosis was based on the presence of fixed Centro facial erythema or phymatous changes or on presence of two or more of the followings, which may be considered as diagnostic: flushing, papules and pustules, telangiectasia and ocular manifestations. There are four rosacea type as erythematotelangiectatic (neurovascular), papulopustular (inflammatory), phymatous, and ocular types. [26] While no such features were seen in GR nor LMDF.In this study, the frequency of GR patients was less common than LMDF, the mean age was comparable with previous reports [7,8] but male predominance (2.5:1) could be due to small sample size. Lesions showed typical facial involvement with Centro facial (forehead, nose and cheeks) and periorificial predilection, which is similar to what had been described, [1-3] but one case with additional lateral side of face involvement, (Fig. 1). No case with extra facial lesions was seen as previously reported. [27] No usual features of rosacea such as background erythema or phymatous changes were seen which is comparable to previous reports. [1-3] Histologically, noncaseating epithelioid-cell granuloma pattern was seen centered on hair follicles, just like what had been reported. [3,5,6] In LMDF cases of present study, the mean age was obviously higher than previously reported, [18,24] although rare reports in elderly and children were mentioned. [19-21] Female gender predominance was clear (1.5:1). Histological examination showed lesion to be in late stage of necrosis with caseating granulomas, giant cells pustules and abscesses embedding the hair follicles with neutrophils seen inside the lesions.In LMDF, the upper and lower eyelids were involved in 10(43.4%) cases versus 4(17.3%) cases of only upper eyelids as opposed to usual emphasis on frequent lower lid involvement. 10 Whereas no cases of eyelid lesions were seen in GR cases.GR and LMDF share many common clinical and histological features as the two conditions are of unknown aetiopathogenesis, have comparable age according to this study, the preferential symmetrical facial and periorificial involvement, [1-3,10] reports of rare extra facial location, [11-15,27] histological granulomatous pattern centered around follicles with absence of the usual necrosis in GR cases and some cases of LMDF, and the negative PCR test for mycobacterium tuberculosis. [24] Many medical literatures focus on whether there is perifollicular involvement in both conditions, but we think this is of little importance as a biopsy of the face, which is rich in hair follicles and in many diseases can show inflammatory reaction around or inside the follicles. On the other hand, there are some differences between them such as caseation necrosis prominence [17,22,23] and the relatively larger nodular more inflammatory lesions in LMDF in the present work present work. The eye involvement was reported in 3-54% of cases of GR. [28]The demodex mite was detected in 25% of GR while not present in LMDF. [24] The natural course of LMDF is spontaneous regression in 12-18 months followed by scarring occasionally [21] while GR has no tendency toward spontaneous involution. [29] The GR responds to classical rosacea treatment such us tetracyclines, while LMDF is often resistant to these standard therapies. Corticosteroids, quite effective in LMDF, generally aggravate the lesions of GR. [5,29] The last (2017) update by the National Rosacea Society Expert Committee didn’t include GR features in their diagnostic nor major criteria, 26 something that raise a question whether GR is a specific entity unrelated to rosacea as proposed by Skowron F. et al. [29]Regarding the present study, we found much difficulty to differentiate between these two conditions whether the clinical or the histopathological features. In addition, there is much confusion about naming these conditions as some authors call LMDF as GR. [30,31]Regarding therapy of both diseases, we found that small dose of oral prednisolone was more effective than ordinary treatment of rosacea.
5. Conclusions
- In the present study and after reviewing literature, we found many sharing and overlapping clinical and histopathological features between LMDF and GR; therefore, both are probably related to one entity, as a twin of the same disease, and not related to ordinary rosacea. Hence, we propose the term “rosoid facial granulomatosis” as an umbrella to include both conditions at the two ends of one spectrum.
Disclosure
- This study was independent and not funded by any drug company or organization.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML