-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2019; 8(3): 45-48
doi:10.5923/j.ajdv.20190803.03

Intralesional Injection of Hyaluronic Acid as a Long Lasting Therapy of Morphea Sclerosis
Khalifa E. Sharquie1, Fatema A. Al-Jaralla2, Inas K. Sharquie3
1Department of Dermatology, College of Medicine, University of Baghdad, Iraqi and Arab Board for Dermatology & Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
2Department of Dermatology, College of Medicine, University of Baghdad, Baghdad, Iraq
3Department of Microbiology and Immunology, College of Medicine, University of Baghdad, Baghdad, Iraq
Correspondence to: Khalifa E. Sharquie, Department of Dermatology, College of Medicine, University of Baghdad, Iraqi and Arab Board for Dermatology & Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Morphea often ends with sclerosis which is difficult to be managed with ordinary therapy like topical and systemic corticosteroids to name a few. Invasive surgical techniques could be used to correct these deformities. Hyaluronic acid (HA) fillers are safe cosmetic agents that could be used to correct depressions and deformities. Objective: is to use high volume HA filler to treat and correct morphea sclerosis and deformities like en coupe de sabre of face. Patients and methods: A total of 16 patients with morphea; seven females and nine males were included; their ages ranged from 8-35years while one female patient with systemic sclerosis age 45y. Intralesional infiltration of sclerosis using hyaluronic acid 26 mg/ml was carried out once monthly for 2 months to ten patients with inflammatory stage morphea; and single injection to six patients with burnt out morphea sclerosis. Follow up was done every 2 weeks for 2 months to 4 years after injection. Two adult males with old ordinary scars, more than 10 years duration, on the hand and forearm were injected with HA and watched for four months for any change in texture and size of scars. Results: In six patients with stable, burnt-out disease and depressed sclerosed lesions, including one patient with linear scleroderma (en coupe de sabre) and one woman with systemic sclerosis with facial involvement; all six patients had obvious improvement that was noticed after two weeks of injection while marked response was seen after about two months and one patient maintained full correction after four years of follow up. Ten patients with active inflammatory morphea showed some improvement at time of injections but relapsed again and did not maintain correction on follow up. The two patients with ordinary scar that was injected with HA did not show changes in size and texture after four months follow up. Conclusion: Intralesional injection of hyaluronic acid is an effective therapy of morphea sclerosis. It acts through stimulation of new connective tissue formation and has long lasting effect. No adverse effects are recorded in any patients, no exacerbation or activation of the original disease. The results of present study can open up new horizons for treatment of morphea using HA fillers.
Keywords: Intralesional hyaluronic acid, New therapy of morphea sclerosis, En coupe de sabre
Cite this paper: Khalifa E. Sharquie, Fatema A. Al-Jaralla, Inas K. Sharquie, Intralesional Injection of Hyaluronic Acid as a Long Lasting Therapy of Morphea Sclerosis, American Journal of Dermatology and Venereology, Vol. 8 No. 3, 2019, pp. 45-48. doi: 10.5923/j.ajdv.20190803.03.
Article Outline
1. Introduction
- Morphea, also known as localized scleroderma, is a disorder characterized by excessive collagen deposition leading to thickening of the dermis, subcutaneous tissues, or both. Morphea is classified into circumscribed, generalized, linear, and pansclerotic subtypes according to the clinical presentation and depth of tissue involvement. [1]Its aetiology is not completely understood and it is estimated that 50% of cases go into remission within 2-7 years of the onset of the disease. [2] Volume loss, bone defects and hemifacial atrophy with paramedian or central ormities of face are associated with cosmetic concern for the patient. [3] Most recent Iraqi study showed that morphea lesions almost always started as pigmented patches that might stay for several months to years before changing into white ivory sclerosed indurated areas. While the histopathological changes in morphea could be classified into early pigmented phase where there was mild acanthosis, increase in basal melanosis, vascular dilation and massive deposition of collagen together in the dermis and panniculus. While in late cases especially with sclerosis there was in addition thinning and atrophy of epidermis with basal liquefaction plus homogenization and hyalinization of collagen in papillary dermis simulating lichen sclerosus et atrophicus. In addition to massive collagen deposition [4].Various methods to augment or reconstruct the volume loss and deformity have been implied including direct resection and suturing, autologous fat grafting, reconstructive flaps [5] and or polyethylene implants. [2,6]Hyaluronic acid (HA) fillers were designed to provide temporary correction to enhance static contour defects. It is now theorised that these HA fillers may have more permanent and longer lasting effects. [7] Wang et al. explored the mechanisms behind potential neocollagenesis from injection of cross‑linked HA fillers. [8] They were able to prove that fibroblasts take on a more morphologically stretched shape and a more active phenotype as a result of HA fillers.Hyaluronic acid (HA) filler is an excellent alternative to invasive procedures for restoring the defect in morphea. As a non-sulfated glycosaminoglycan that naturally occurs in the body, HA has been shown to hydrate, volumize, and stimulate collagen synthesis. [8] Initially introduced for intra-articular joint injection, and then HA fillers have become the most commonly used facial fillers over the past several years. They exhibit an excellent safety profile and are simple to use, allergy free, and temporary. In cases of poor results or complications, the enzyme hyaluronidase can be used to catalyze the degradation of hyaluronic acid. [9]Herein, we have treated a total of 16 patients with morphea with hyaluronic acid filler while the disease activity was being treated and maintained with topical and systemic steroids and oral zinc sulfate.
2. Patients and Methods
- A total of 16 patients with morphea, seven females and nine males were included during the period between 2008 and 2019; their ages ranged from 8-35 years while one female patient with systemic sclerosis age 45y. Only two patients were married, the other patients were unmarried.All patients had wide spread morphea of average 3yrs duration; and treated with systemic and topical steroids and oral zinc sulphate; they had areas of sclerosis that did not resolve by this therapy, they were enrolled because they sought contour restoration. One patient aged 8yrs old was presented with sclerosis due to en coup de sabre; he had no active inflammatory lesions.Intralesional infiltration of sclerosis using hyaluronic acid 26 mg/ml of Neauvia organic (www.neauvia.com) was carried out once monthly for 2 months to ten patients with inflammatory stage morphea; and single injection to six patients with burnt out morphea sclerosis. HA filler was injected deep into dermis including the scarred area using cannula for sclerosis and fine needle for the inflammatory lesions. There were no complications of the procedure. Contour deformity was well corrected in all patients with good patient satisfaction at the time of injection. Follow up was done every 2 weeks for 2 months to 4 years after injection. Photographs were taken before and after therapy to compare the results. Two adult males with old ordinary trauma scars, more than 10 years duration, on the hand and forearm were injected with HA and watched for four months for any change in texture and size of scars.
3. Results
- In six patients with stable, burnt-out disease and depressed sclerosed lesions, including one patient with en coup de sabre (Fig 1), and one woman with systemic sclerosis with facial involvement; all six patients had obvious improvement was noticed after two weeks of injection, marked response was seen after about two months (Fig 2), and one patient maintained full correction after four years of follow up. (Fig 1, 3, 4 and 5)
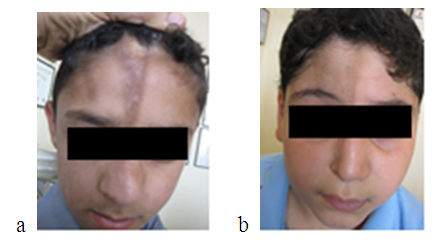 | Figure 1. a - eight years old boy with linear scleroderma (en coupe de sabre) before and b-4 years after hyaluronic acid injection showing marked cosmetic improvement |
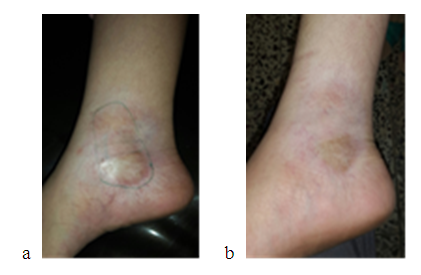 | Figure 2. a -female patient with morphea on right ankle before and b-two months after hyaluronic acid injection showing obvious reduction of sclerosis |
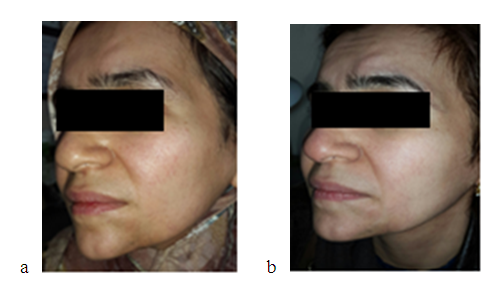 | Figure 3. a -35 years old woman with morphea on chin before and b-nine months after hyaluronic acid injection showing good cosmetic improvement |
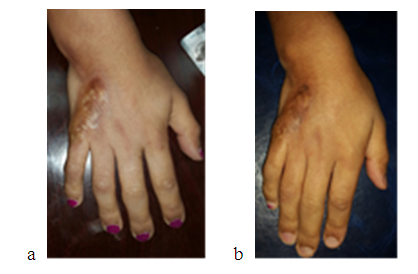 | Figure 4. a -showing female patient with morphea sclerosis on right hand before and b-two months after hyaluronic acid injection |
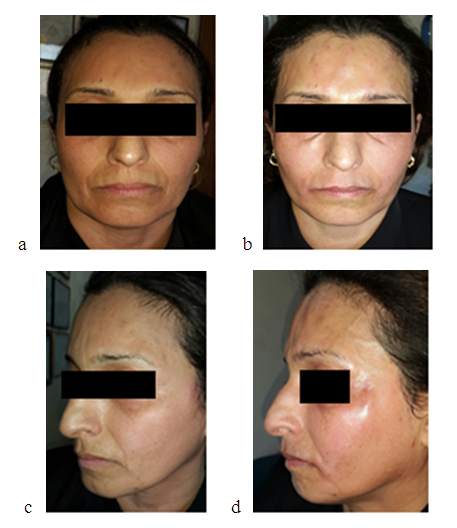 | Figure 5. a - Showing 45 year’s old woman with systemic sclerosis before and b-after hyaluronic acid injection. C-lateral view of the same patient before and d- after hyaluronic acid injection |
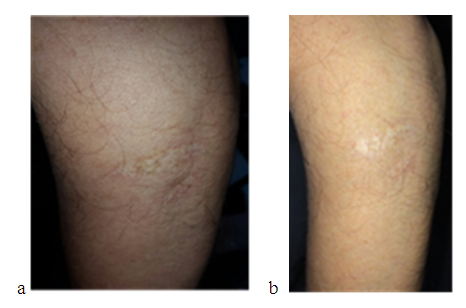 | Figure 6. Ordinary trauma scar treated with HA before (a) and (b) after 4 months after injection showing no obvious change in scar |
4. Discussion
- Morphea, also known as localized scleroderma, is a rare fibrosing disorder of the skin and underlying tissues but seems to be not uncommon problem in Iraq [4]. The length of the active, inflammatory stage typically ranges from 2 to 5 years [10,11]. Attempts at halting the progression during this phase have led to the use of a variety of pharmacologic therapies, including topical, intralesional, or systemic glucocorticoids, antimalarials, retinoids, penicillamine, penicillins, phenytoin, griseofulvin, calcitriol, interferon, and methotrexate. Other modalities such as phototherapy and physiotherapy have also been employed [12-16]. The personal experience of Sharquie in the past 25 years by using topical and systemic corticosteroid with oral zinc sulfate for inflammatory stage and subcision for morphea sclerosis especially the en coupe de sabre; had shown encouraging results. However, many patients refuse surgery and prefer more conservative options to correct the deformity. Hence the idea to use HA fillers to correct the deformities was studied.Hyaluronic acid is an anionic, non-sulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. Its main advantage is identical molecular structures in all living organisms, which doesn’t lead to immunogenicity. Crosslinked hyaluronic acid is ideal to fill soft tissues, since it reduces the rate of degradation and retains high water absorption [17]. There are no reports in the literature on the relationship between the use of hyaluronic acid and the development of autoimmune diseases, such as dermatomyositis, systemic lupus erythematosus, rheumatoid arthritis, and progressive systemic sclerosis [18].Currently, the consensus of world experts in dermatology, found no absolute contraindication to use hyaluronic acid in cases of scleroderma [19]. Faster degradation of filler in morphea was one of our greatest concerns which were not seen in six patients due to inactive nature of the disease. In contrast to ten patients who had no benefit of HA filler probably due to active disease with little sclerosis. The HA fillers are a good minimally invasive temporary therapeutic option for volume defect correction in focal circumscribed morphea. It was demonstrated and theorised that existing collagen fibers are stretched by HA injection, which imposes mechanical tension on surrounding fibroblasts, therefore, stimulating them to increase production of Types 1 and 3 collagen. Procollagen, MMP and MMP tissue inhibitors of metalloproteinase (TIMP-1) were also measured by ELISA and quantitative PCR. They also found a statistically significant increase in procollagen, TIMP-1 and gene expression of procollagen 1 and 3, 1 month after injection. Procollagen levels remained elevated at three and 6 months of subsequent injections [20].Treatment of the en coup de sabre variant of linear morphea has proved challenging in regard to obtaining disease remission and improving clinical appearance. The first case treated by Sharquie with HA filler was an 8yr old boy with en coup de sabre sclerotic stage. The patient maintained full correction after four years of single injection. This encouraged Sharquie to use HA fillers for morphea lesions of 15 other patients. The results were satisfactory in patients with burnt out stage, in contrast to patients with active morphea in whom the results were not maintained probably due to the ongoing inflammatory process that interfered with fibroblasts to produce new collagen. The limitation of this study was the small number of the patients enrolled.In summary, we feel that hyaluronic acid filler may be safely and successfully used as monotherapy for cosmetic improvement of localized morphea lesions. The benefit is most prominent in well-selected patients who may experience atrophy but in whom the disease is burnt out.
5. Conclusions
- Intralesional injection of hyaluronic acid is an effective long lasting therapy of morphea sclerosis. It acts through stimulation of new connective tissue formation. No adverse effects are recorded in any patients. No exacerbation or activation of the original disease. Currently, autoimmune diseases aren’t contraindications to use fillers. The results of present study can open up new horizons for treatment of morphea using HA fillers. From this study, we conclude that the intralesional injection of hyaluronic acid is an effective long lasting therapy of morphea scleroses.
DISCLOSURE
- This study was an independent study and not funded by any drug companies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML