-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2017; 6(3): 51-57
doi:10.5923/j.ajdv.20170603.02

In vitro Hair Growth Promoting Effects of Naringenin and Hesperetin on Human Dermal Papilla Cells and Keratinocytes
Alka Madaan1, Vidushi Joshi1, Amitesh Kishore1, Ritu Verma1, Anu T. Singh1, Manu Jaggi1, Young Kwan Sung2
1Cell Biology Lab, Dabur Research Foundation, Sahibabad, Ghaziabad, Uttar Pradesh, India
2Department of Immunology and Hair Research Center, School of Medicine, Kyungpook National University, Daegu, Korea
Correspondence to: Alka Madaan, Cell Biology Lab, Dabur Research Foundation, Sahibabad, Ghaziabad, Uttar Pradesh, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Alopecia (hair loss) is a common and distressing condition. Therefore, it is important to develop new therapeutic agents to prevent the hair loss and maintain hair growth. Natural compounds, such as flavonoids are known to possess hair-growth promoting properties. The hair growth promoting effects of Naringenin and Hesperetin and their in vitro biological effects on human Dermal Papilla cells (DPCs) and keratinocytes (HaCaT cells) have not been reported yet to the best of our knowledge. In the present study, we have investigated the in vitro effects of Naringenin and Hesperetin on key constituent cells of hair; DPCs and keratinocytes. Cellular proliferation in DPCs and HaCaT cells was determined by MTT assay. Vascular Endothelial Growth Factor (VEGF) levels were estimated by ELISA. Naringenin and Hesperetin significantly increased the proliferation of DPCs and HaCaT cells. Restoration of cell viability against Hydrogen peroxide (H2O2) induced cytotoxicity suggested protection against oxidative stress associated with hair. Stimulation of VEGF secretion was also observed. The preliminary results obtained here suggest the hair growth promoting properties of Naringenin and Hesperetin, presenting them as potential candidates for natural hair-growth promoting compositions. Further, the effect of compounds in ex vivo and in vivo models will substantiate their hair growth promoting properties.
Keywords: Dermal Papilla Cells, Keratinocytes, Proliferation, Oxidative stress, VEGF
Cite this paper: Alka Madaan, Vidushi Joshi, Amitesh Kishore, Ritu Verma, Anu T. Singh, Manu Jaggi, Young Kwan Sung, In vitro Hair Growth Promoting Effects of Naringenin and Hesperetin on Human Dermal Papilla Cells and Keratinocytes, American Journal of Dermatology and Venereology, Vol. 6 No. 3, 2017, pp. 51-57. doi: 10.5923/j.ajdv.20170603.02.
Article Outline
1. Introduction
- Hair is a specialized derivative structure of skin and a defining characteristic of human integumentory system. Root of the hair is buried inside the epidermis and enclosed within a hair follicle, consisting of epithelial components (matrix and outer-root sheath) and dermal components (dermal papilla and connective tissue sheath) [1]. The dermal papilla (DP) is located at the base of hair follicle and functions as primary mesenchymal component of hair. It plays pivotal role in hair cycle by induction of new hair follicles and maintenance of hair growth [2]. Dermal Papilla cells (DPCs) are rich source of nutrients and secrete multiple growth factors, which support proliferation and growth of keratinocytes [3]. In addition to DPCs, root sheath cells and melanocytes of the scalp follicle, the keratinocytes play a crucial role in hair growth as the epithelial-counterpart cells [4]. The hair fiber is composed of 10 nm keratin filaments produced by keratinocytes in hair follicle [5]. Angiogenesis is a key event during hair follicle cycling. Follicle-derived Vascular Endothelial Growth Factor (VEGF) promotes perifollicular vascularization, which restores the oxygen supply to tissues for hair growth [6].Hair loss is a common hair disorder, especially in males. The causes of alopecia include aging, nutritional deficiency, hormone imbalances, disease and stress. Oxidative stress induced by the environment, aging and stress is responsible for the onset and progression of hair loss. Reactive oxygen species (ROS) are key etiological factors for the senescence of DPCs. Levels of ROS, such as H2O2 are significantly enhanced during stressful conditions (Ultraviolet radiation, drugs, smoke) [7]. Finasteride and Minoxidil have been approved for treatment of hair loss by the US Food and Drug Administration (FDA). However, due to their limited efficacy and side effects, development of natural hair growth promoters is an unmet need of the hour. Natural agents promote hair growth by stimulating proliferation of DPCs and keratinocytes [8, 9].Flavonoids have been reported to possess hair growth promoting potential. However, only limited reports are available in literature, where mechanistic action of flavonoids as hair growth promoters is studied in DPCs. Baicalin enhanced DPCs proliferation and hair growth in mice [10]. Epigallocatechin-3-gallate (EGCG) stimulated DPCs proliferation in vitro [11]. It also protected human DPCs against Dihydrotestosterone by altering the miRNA expression [12]. Quercetin promoted in vivo hair growth in C3H/HeJ mouse model [13]. Naringenin and Hesperetin (flavanone subgroup of flavonoids) possess various pharmacological properties, such as anti-inflammatory and analgesic potential [14]. The mechanism of hair growth promoting potential of these 2 compounds has not been elucidated in detail. Takahashi et al. have evaluated the effect of Hesperetin on murine hair epithelial cells [15]. However, the hair growth promoting effects of Naringenin and Hesperetin in human Dermal Papilla cells from hair follicle and keratinocytes (HaCaT cells) are yet to be investigated. Hence, in the present study, we examined their effects on in vitro cell proliferation, protective effect against oxidative damage and Vascular Endothelial Growth Factor (VEGF) secretion in human Dermal Papilla cells (DPCs) from hair follicle and keratinocytes (HaCaT).
2. Materials and Methods
2.1. Chemicals/Regents
- Dulbecco’s modified eagle’s medium (DMEM) and Fetal Bovine Serum (FBS) (Invitrogen, Gibco), Penicillin/ Streptomycin (Himedia) were used in the study. Trypsin, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), Dimethylsuploxide (DMSO), Hydrogen Peroxide (H2O2), Naringenin and Hesperetin were purchased from Sigma. Human VEGF ELISA kit (Quantikine, R&D systems, Minneapolis, MN, USA), Minoxidil Sulphate (Clearsynth, India) were used in the study.
2.2. Preparation of Stock Solutions of Compounds
- Test compounds (Naringenin and Hesperetin) were dissolved in DMSO to prepare the stock solution of 20 mM. These stock solutions were further diluted in serum free medium (SFM) to achieve final concentrations in the range of 0.001 µM – 10 µM. Final DMSO in cells did not exceed 0.05% (v/v). DMSO treated cells were used as control. Minoxidil Sulphate was dissolved in sterile SFM to prepare stock solution of 10 mM.
2.3. Cell Lines
- Immortalized human dermal papilla cell line; SV40T-hTERT-DPC (simian virus 40T- transformed scalp DPCs transfected with hTERT) as developed previously, was used in the study [16]. DPC line was maintained in DMEM with 10% FBS with Penicillin/Streptomycin at 37C, 5% CO2 and 95% humidity and subcultured by trypsinization upon attaining 70 – 80% confluence. Immortalized human keratinocyte cell line (HaCaT) was procured from National Centre for Cell Sciences (NCCS), Pune, India and maintained in DMEM with 10% FBS with Penicillin/Streptomycin at 37C, 5% CO2 and 95% humidity. HaCaT cell line was used within passage No 10.
2.4. Proliferation Assay
- DPCs (1,000 cells/well) were plated in 96-well culture plates in DMEM + 10% FBS and incubated in a CO2 incubator for 24 h. Cells were serum-starved by incubating in medium without FBS for another 24 h and treated with Naringenin and Hesperetin in the concentration range 0.001 µM – 10 µM for 5 days. Cells treated with Minoxidil Sulphate were included as positive control (PC). HaCaT cells (2500 cells/well) were plated in 96-well culture plates in DMEM + 10% FBS and incubated in a CO2 incubator for 24 h. After serum-starvation, HaCaT cells were treated with Naringenin and Hesperetin for 48 h. Cells were also treated with Minoxidil Sulfate (PC). Effect on cell proliferation was determined by MTT assay. MTT (5 mg/ml) was added to wells and incubated at 37C for 3 h. The supernatant was aspirated and DMSO was added to dissolve formazan crystals. The absorbance of each well was measured at 540 nm using Multi-Mode Microplate Reader. Effect on cell-proliferation was calculated as: % Cellular proliferation = {(X-Y)/Y} x 100; where X = Absorbance of cells treated with compounds, Y= Absorbance of control cells.
2.5. Cell Viability against Oxidative Damage
- DPCs (5,000 cells/well) and HaCaT cells (10,000 cells/well) were plated in 96-well culture plates in DMEM + 10% FBS for 24 h. Cells were co-treated with H2O2 (250 µM) and Naringenin and Hesperetin. Cells were also treated with Minoxidil Sulfate (PC). Survival of cells against H2O2 induced cytotoxicity was monitored after 48 h by MTT assay. Protective effect against H2O2 induced cell death was determined as restoration of cell viability:% Cell viability = 100 - (% Cytotoxicity),where, % Cytotoxicity = {(A-B)/A} x 100; where A = Absorbance of Untreated cells, B = Absorbance of cells treated with H2O2 alone or H2O2 + compounds.
2.6. VEGF Estimation
- DPCs (0.1 million cells/well) and HaCaT cells (0.2 million cells/well) were plated in 6-well culture plates in DMEM + 10% FBS for 24 h. After sera-starvation, cells were treated with Naringenin and Hesperetin for 24 h. Cells were also treated with Minoxidil Sulphate (PC). Secretion of VEGF into extracellular medium was determined by ELISA (R&D systems). Effect on VEGF secretion was calculated as: %Increase in VEGF = {(C-D)/D} x 100; where C = Concentration of VEGF (pg/ml) in cells treated with compounds, D = Concentration of VEGF (pg/ml) in control cells.
2.7. Statistical Analysis
- Experimental values were expressed as arithmetic mean ± SEM. Statistical differences between control and treatment groups were determined using One-way ANOVA with Dunnett’s multiple comparison post test. Student’s t-test was used to determine statistical differences between H2O2 treated group vs. untreated.
3. Results and Discussion
3.1. Effect on Cell Proliferation
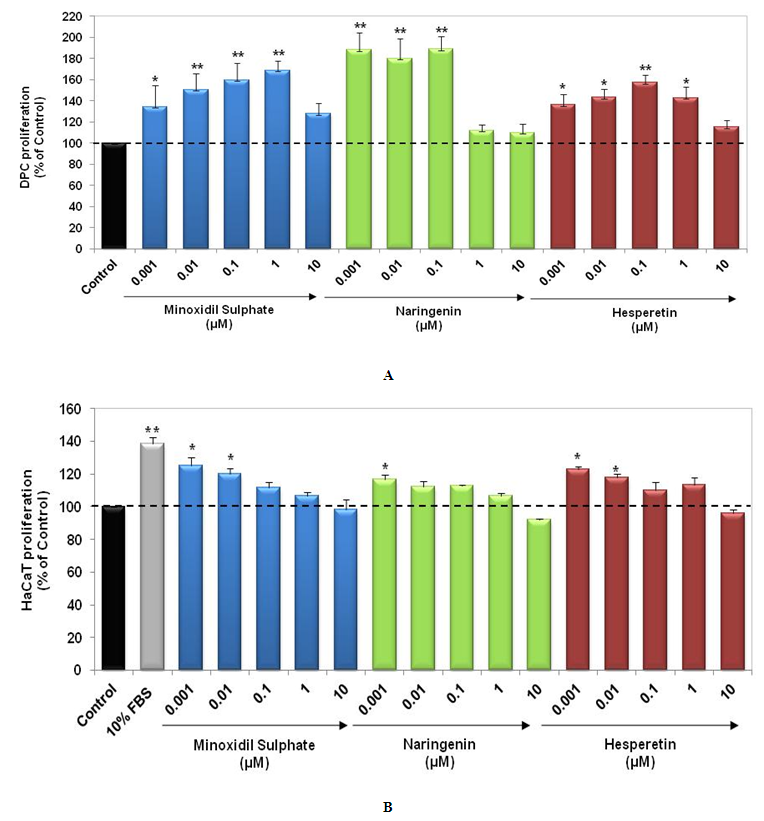
- In this study, we examined the effect of Naringenin and Hesperetin on proliferation of DPCs and HaCaT cells. DPCs and HaCaT cells have been previously used as target cell populations to study hair growth potential of compounds in vitro [4, 9, 17]. Our results demonstrated that Naringenin and Hesperetin induced significant proliferation of DPCs and HaCaT cells as compared to untreated cells. Naringenin resulted in significant (p<0.01) increase in proliferation of DPCs at 0.001, 0.01 and 0.1 µM. Hesperetin also led to enhancement of DPCs proliferation at 0.001 µM - 1 µM (p<0.05, p<0.01) (Figure 1a). In addition, Naringenin and Hesperetin (0.001 µM - 1 µM) also enhanced proliferation of keratinocytes; HaCaT cells (Figure 1b). Minoxidil Sulphate (PC) showed increase in proliferation of DPCs and HaCaT cells. Stimulatory action of Naringenin and Hesperetin on proliferation of DPCs and HaCaT cells indicates their potency as hair growth promoters.
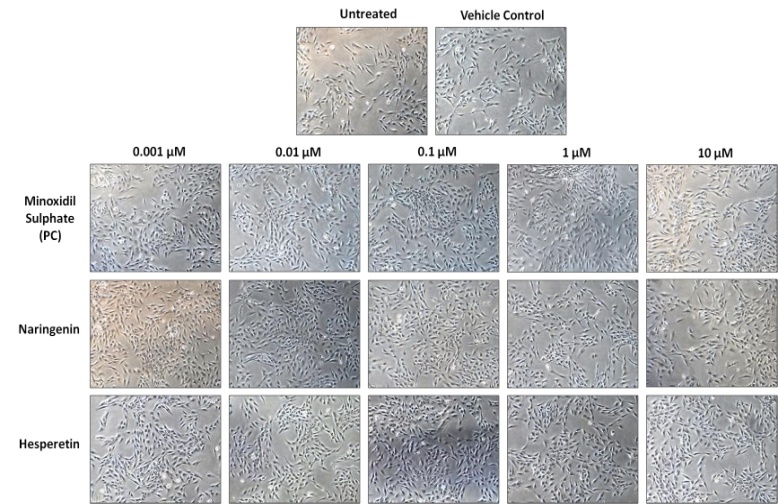 | Figure 2. Morphology of DPCs treated with Naringenin and Hesperetin for 5 days (Magnification – 100X) |
3.2. Protection against Oxidative Damage
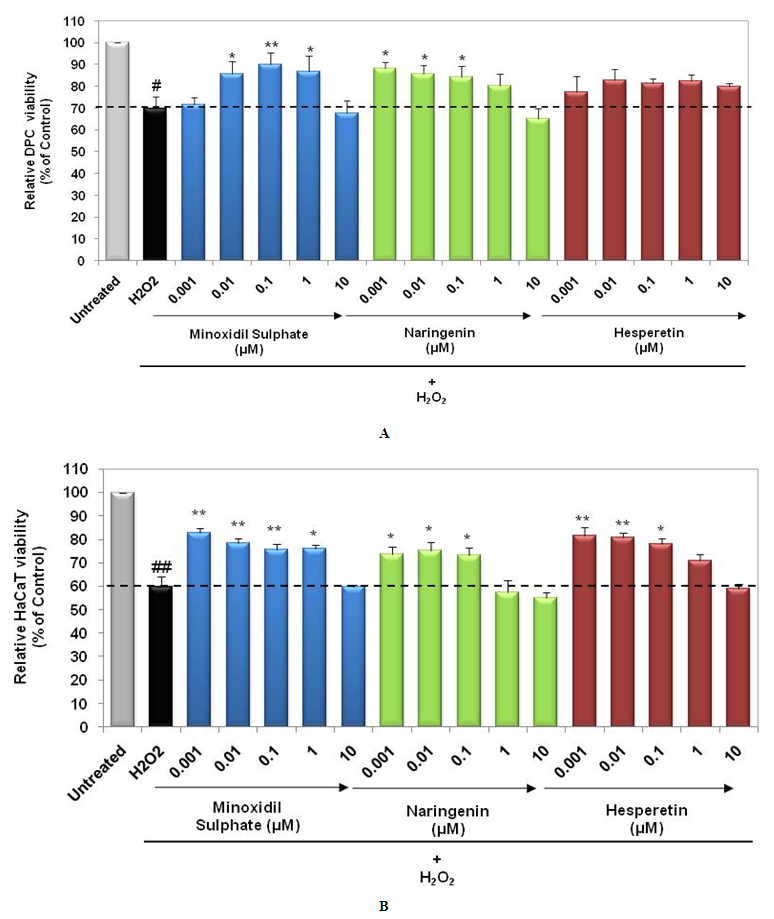
- Oxidative stress has been identified as an important etiological factor for the induction of dermal papilla cell senescence and cell death [18]. Arctiin, a lignin chemical reagent isolated from Arctium lappa has demonstrated anti-oxidative effects on human hair dermal papilla cells (HHDPCs) against H2O2 induced cytotoxicity [19]. We assessed protective effect of Naringenin and Hesperetin against H2O2 induced cell death in DPCs and HaCaT cells. Interestingly, both the compounds were able to show protective effect by restoration of DPCs and HaCaT cells viability. Naringenin (0.001 µM – 0.1 µM) showed significant (p<0.05) protection against H2O2 induced death of DPCs as well as HaCaT cells (Figure 3a and 3b). Hesperetin also restored viability of DPCs as compared to H2O2 induced damage. In HaCaT cells, Hesperetin at 0.001, 0.01 and 0.1 µM led to significant (p<0.01, p<0.05) restoration of cell viability. Minoxidil Sulphate (PC) showed protection against oxidative damage in DPCs and HaCaT cells. These results suggest that Naringenin and Hesperetin can prevent damage in DPC and keratinocytes mediated by oxidative stress and hence help in the maintenance of hair growth.
3.3. Effect on VEGF Secretion
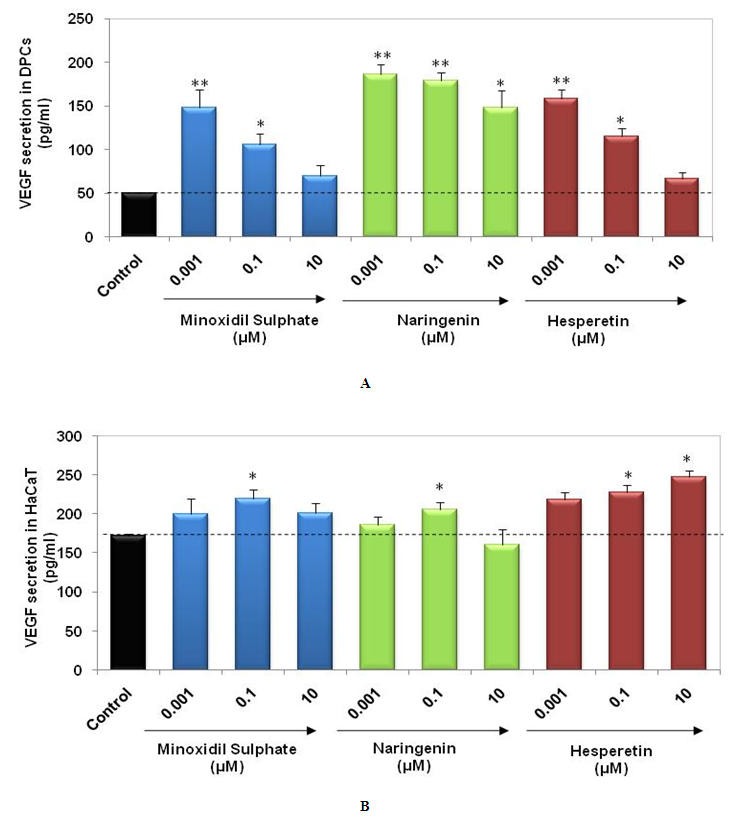
- VEGF is known to play an important role in mediating angiogenesis during hair growth cycle [20]. Stimulation of VEGF by DPCs and HaCaT supports the hair growth promoting potential of herbal extracts [4, 21]. Naringenin and Hesperetin (0.001µM – 10 µM) were found to be non-cytotoxic in DPCs and HaCaT cells after 24 h (Data not shown). Hence, we assessed effect of these 2 compounds on VEGF secretion in DPCs and HaCaT after 24 h of treatment. We observed that Naringenin and Hesperetin resulted in increased secretion of VEGF from DPCs and HaCaT cells (Figure 4a-4b). Treatment of DPCs with Naringenin and Hesperetin at 0.001, 0.1 and 10 µM resulted in significant increase in VEGF secretion (p<0.01, p<0.05). Naringenin at 0.1 µM and Hesperetin at 0.1 and 10 µM induced significant (p<0.05) increase in VEGF secretion by HaCaT cells. Minoxidil Sulphate (PC) demonstrated stimulation of VEGF in DPCs and HaCaT cells. Stimulatory effect on VEGF secretion in both DPCs and HaCaT cells strengthens the hair growth promoting potential of Naringenin and Hesperetin.
4. Conclusions
- In summary, compounds with dual activity on key cell populations of hair, DPCs and keratinocytes, present as promising hair growth promoters. The 2 flavonoids tested here; Naringenin and Hesperetin have demonstrated stimulatory effects on the in vitro proliferation of DPCs and HaCaT cells, in basal as well as H2O2 induced oxidative damage model. Enhancement of VEGF secretion was also observed, supporting hair growth associated angiogenesis. Taken together, the preliminary results obtained by us suggest the possible hair growth promoting potential of Naringenin and Hesperetin, presenting them as potential candidates for natural hair-growth promoting compositions. Further, it is required to study the effect of compounds in ex vivo and in vivo models of hair growth. This will help in elucidation of mechanism of action and substantiation of hair growth promoting properties of Naringenin and Hesperetin.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML