-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2017; 6(1): 6-10
doi:10.5923/j.ajdv.20170601.02

A Comparative Study to Test a Plant Extract against Minoxidil in HaCaT Cells
Murat Türkoğlu, Cihat Dündar, Hakan Sevinç, Songül Kılıç
Biota Laboratories R&D Center, Sancaktepe, Istanbul, Turkey
Correspondence to: Murat Türkoğlu, Biota Laboratories R&D Center, Sancaktepe, Istanbul, Turkey.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

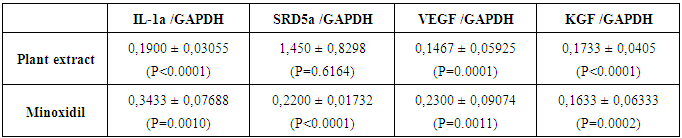
Topical 5% minoxidil solution twice a day was reported to be effective in many types of hair loss. However, some plant extracts showed a similar anagen prolongation effect in clinical studies. In this study, anti-hair loss drug minoxidil was compared to a botanical extract using HaCaT cells to investigate the cellular response to both treatments. HaCaT cells were incubated at 1% concentration of both minoxidil solution and the botanical extract. Total RNA isolation, cDNA synthesis and the gene expression analysis (RT-qPCR) were performed.The plant mixture of the botanical extract was as follows: Chamomilla recutita, Urtica urens, Urtica dioica, Equisetum arvense, Achillea millefolium, and Ceratonia siliqua. Results of the gene expression analysis showed that the botanical extract and minoxidil solution caused significant downregulation of interleukin-1α (IL-1α) gene expression. The botanical extract treatment resulted in five-fold decrease in the IL-1α gene expression and minoxidil treatment resulted in a three-fold decrease. Stereoid-5α-reductase type 2 (SRD5α2) gene expressions was not significantly affected by the botanical extract. However, it was also downregulated after minoxidil treatment. It was hypothesized that anti-hair loss effect of minoxidil could be due to the suppression of IL-1α and on SRD5α gene.
Keywords: Hair Loss, Minoxidil, Interleukin 1-α, 5-α reductase type 2, Real-time PCR, Herbal Extract
Cite this paper: Murat Türkoğlu, Cihat Dündar, Hakan Sevinç, Songül Kılıç, A Comparative Study to Test a Plant Extract against Minoxidil in HaCaT Cells, American Journal of Dermatology and Venereology, Vol. 6 No. 1, 2017, pp. 6-10. doi: 10.5923/j.ajdv.20170601.02.
Article Outline
1. Introduction
- Interleukin (IL-1) was shown to be a potent inhibitor of hair growth. Furthermore, it was reported to be a significant factor in alopecia areata (AA) which is a disease of the anagen stage hair follicles, considered to be autoimmune in origin [1, 2]. In isolated hair follicles IL-1β decreased hair growth approximately 60-80%. This hair growth inhibition was mediated by cAMP 2. IL-1α and IL-1β share common receptors. Rückert, et al. [3] reported that high dose proinflammatory cytokines such as IL-1β induced apoptosis of hair bulb keratinocytes in vivo. The hair loss that is seen in transgenic mice overexpressing IL-1α in the epidermis and outer root sheath was reported and compatible with the view of inflammatory cytokines may induce apoptosis within the hair follicle epithelium [4]. In AA a more serious disease was encountered in patients who show gene polymorphism and have insufficient amount of IL-1 receptor antagonists [2]. There were many publications on the mechanism of action of minoxidil. Topical 5% minoxidil solution twice a day on 47 patients was reported to be effective in AA [5]. Minoxidil’s effect on stimulating hair growth does not depend on either a hormonal factor or on the inhibitor action on 5α-reductase. It was also reported that minoxidil increased the mRNA expression for the VEGF at the dermal papilla level [6]. In cell cultures, minoxidil extend the survival and slowed the aging of human keratinocytes and it acts on follicular matrix cells extending the anagen phase [7, 8]. When considering the well documented effects of minoxidil on hair growth it is logical to use it as a reference for comparison purposes of any other formulation to promote hair growth. We developed a botanical extract and carried out a clinical study to show its hair growth promoting effects [9]. Chamomilla recutita was reported to contain apigenin as an active ingredient [10] whereas Urtica urens and Urtica dioica were a source of kaempferol, myricetin, linoleic acid [11], oleic acid [12], iron [13] and beta-sitosterol [14]. Equisetum arvense was reported to contain apigenin, kaempferol and beta-sitosterol [15]. Together with apigenin and beta-sitosterol, Achillea millefolium contained folic acid and several minerals [16] as active ingredients. Ceratonia siliqua, on the other hand, contained, tocopherol, thiamine, riboflavin, pantothenic acid, folic acid, zinc, copper, iron as some of the ingredients [17]. Among these active ingredients apigenin, kaempferol, myricetin, linoleic acid, oleic acid, iron and zinc were reported to stimulate hair growth [18]. In this study, we compared minoxidil and the experimental extract in the human keratinocyte cell line (HaCaT).
2. Materials and Methods
2.1. Cell Culture
- The human keratinocyte cell line (HaCaT) was cultured in Dulbecco’s Modified Eagle’s medium (DMEM) with high glucose supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2mM l-glutamine and 100U/ml gentamicin. Cells were maintained at 37°C in a humidified atmosphere at 5% CO2 in Newbrunswick incubator. All supplements and media were purchased from Sigma Aldrich.
2.2. Preparation of Minoxidil Solution
- 522.5 mg minoxidil was dissolved in 25 ml distilled water - 25 ml ethanol mixture to get 5mM minoxidil solution. This solution is used as 100% sample and other concentrations (10%, 5%, 3%, 1% and 0.2%) of the solution were prepared by dilution with distilled water.
2.3. Preparation of Plant Extract
- The plants were obtained from a commercial plant supplier (Martin Bauer Group, Germany). Chamomilla recutita (Chamomile flowers), Urtica urens (Small nettle leaves), Urtica dioica (nettle leaves), Equisetum arvense (Horsetail leaves), Achillea millefolium (Common yarrow flowers), Ceratonia siliqua (Carob fruit) were used as the plants for extract. Dried plants were fine-cut. Forty grams of plant mixture was extracted with 500 mL distilled water for 3 hours at 100°C using a soxhlet extractor. The extract was filtered through a 0.22 µm filter.
2.4. XTT Cell Proliferation and Cytotoxicity Assay
- The Cell Proliferation Kit II (Roche XTT) was used for cytotoxicity assay. This assay based on the cleavage of XTT by metabolic active cell, resulting in an orange formazan dye. The amount of orange formazan dye is quantitated using microplate reader. Briefly, HaCaT cells were seeded into 96-well plates (1x104 cells/well) and were subjected to different concentrations (100%, 10%, 5%, 3%, 1% and 0,2%) an of minoxidil solution and botanical extract. After 72 hour incubation period, 50 µl XTT and activator reagents were added to the each plates according to the manufacturer’s instructions. Then, cells were incubated at 37°C for 4 hours in order that XTT reagent was reduced to formazan compound. The absorbance was read in BIO-RAD microplate reader (Japan) at 450 nm subtracting the background measurement of 620 nm.
2.5. RNA Isolation
- HaCaT cells were incubated with 1% concentration of both minoxidil solution and the botanical extract before total RNA isolation. Total RNA was extracted by using TRI-reagent according to manufacturer’s instructions (Sigma Aldrich). The concentration and purity of isolated RNA samples were determined by measuring optical densities at 260 nm and 280 nm using BioSpec- nano (Japan).
2.6. Reverse Transcription
- Roche Transcriptor First Strand cDNA Synthesis Kit was used for reverse transcription. cDNA synthesis was performed with 500 ng total RNA, 2 µM final concentration of gene specific primers of VEGF, KGF, IL-1α, SRD5α type 2 and GAPDH (Integrated DNA Technologies), 10 U of Transcriptor Reverse Transcriptase, 20 U of Protector RNase Inhibitor, 1mM each of dNTP mix and Transcriptor Reverse Transcription Buffer (5X) according to the manufacturer’s instructions (Roche).
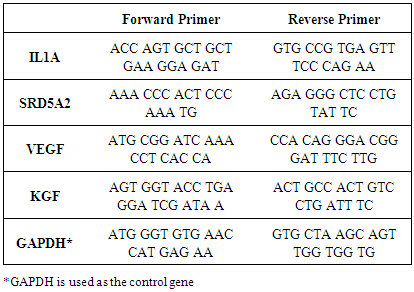
2.7. Real-Time Quantitative Polymerase Chain Reaction
- Real-time PCR (RT-qPCR) reaction was carried out in Light Cycler 96 (Roche) and Fast Start DNA Green Master Kit (Roche) was used. Briefly, total volume of reaction mix was 20 µl containing 10 µl SYBR Green Master Mix (2X), 0.5 µM of reverse and forward primers (Table 1), 2.5 ng cDNA and appropriate amount of nuclease free water. All samples were run as triplicates in each run including a non-template control and four standards (1:10, 1:100 and 1:1000). The PCR parameters were determined separately for each target according to melting and annealing temperatures of primers. Each parameter includes a pre-incubation step for 10 min at 95°C and followed by 45 cycles of 3-step amplification and melting step. Melting curve analysis was performed to verify specificity. Three repeats of gene expression analysis were done (n=3). For quantitation of RT-qPCR results, ΔΔCt method was used (2-ΔΔCt).
|
2.8. Statistical Analysis
- All data are representative of three experiments and expressed as mean ± standard error of the means (SEM). Statistical evaluation was performed by Unpaired t-test using Graph Pad Prism 5 Software (USA) and the results with p value less than 0.05 were accepted as significant.
3. Results and Discussion
3.1. Cytotoxicity Analysis
- Minoxidil solution and the botanical extract showed cytotoxic effect for HaCaT cells at high concentrations. For the subsequent analysis, the possible highest concentration was determined to be 1% (Figure 1).
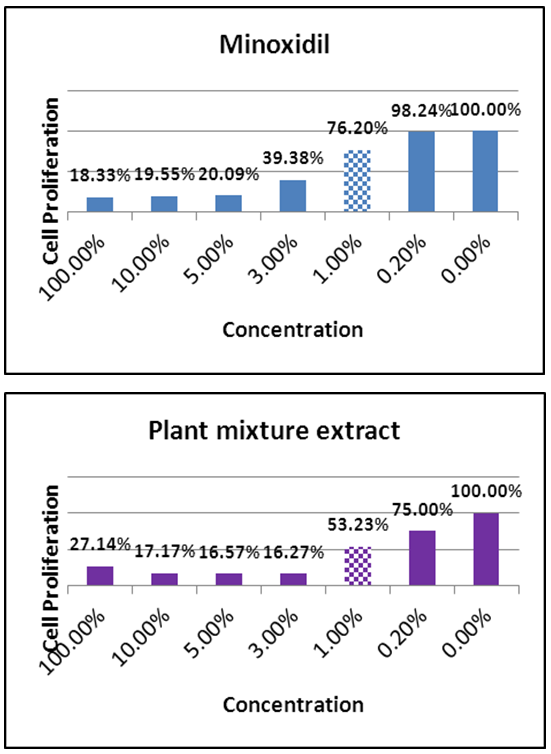 | Figure 1. Cytotoxicity analysis results of minoxidil solution and the plant extract (Dashed bars represent the concentrations chosen for incubation.) |
|
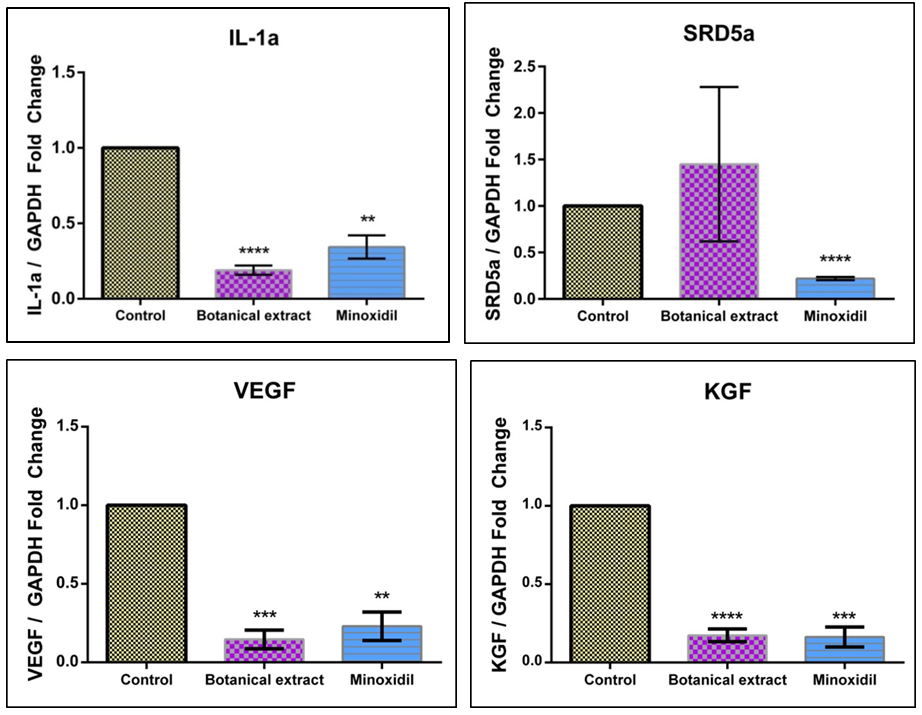 | Figure 2. Gene expression levels of IL-1a, SRD5a2, VEGF, and KGF upon plant extract or minoxidil treatment |
4. Conclusions
- By taking all those into consideration, it was our hypothesis that botanical extracts consisting of different plants can be effective against hair loss. However, their effectiveness must be evaluated by placebo-controlled clinical studies as well as further cell, tissue and organ culture studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML