-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
p-ISSN: 2332-8479 e-ISSN: 2332-8487
2016; 5(3): 51-57
doi:10.5923/j.ajdv.20160503.03

Expression of CD30, CD57 and CD99 Leukocyte Antigens in Eczematous Dermatitis
Mahmoud R. Hussein1, Hanan A. Assaf2, Eman E. Abu-Dief3, Amira A. Abdel-Motaleb4, Asmaa Mahmoud Ahmed1, Abeer Refaiy1
1Pathology Department, Assuit University, Assuit, Egypt
2Dermatology Department, Sohag University, Sohag, Egypt
3Histology Department, Sohag University, Sohag, Egypt
4Dermatology Department, Assuit University, Assuit, Egypt
Correspondence to: Mahmoud R. Hussein, Pathology Department, Assuit University, Assuit, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

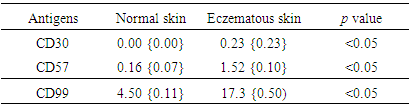
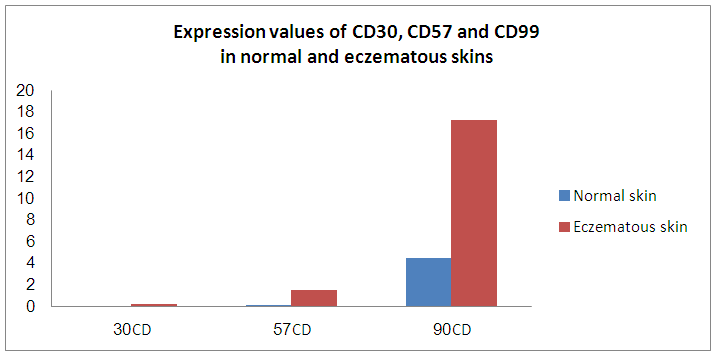
Background: CD30 is a protein that belongs to the tumor necrosis factor receptor superfamily. Its expression was observed in increasing numbers of cutaneous inflammatory conditions. Natural killer cells (CD57 positive cells) have an important role in several inflammatory conditions of the skin. CD99 is a long-known leukocyte antigen expressed at the endothelial cell contacts. It participates in the transendothelial migration of monocytes into the inflamed skin tissue in mice. Objectives: To examine the presence of lymphocyte-surface antigens including CD30, CD99, and CD57 in skin lesions of eczematous dermatitis and to compare the presence of the same molecules in the normal skin. Materials and methods: The presence of these molecules was immunohistologically evaluated in biopsies of normal and lesional skin (50 specimens). Biopsies were obtained from 50 patients suffering from chronic dermatitis. Ten more skin biopsies (normal skin) were included as a control group. Results: In eczematous dermatitis skin, CD30+, CD57+ NK and CD99+ cells were seen in the dermis. Compared to normal skin, there was a statistically significant higher average numbers of these molecules (on the histiocytes and lymphocytes) in the dermis of eczematous skin [CD30: 0.0 (SEM, 0.0) versus 0.23 (SEM, 0.0); CD57: 0.16 (SEM, 0.07) versus 1.52 (SEM, 0.10) and CD99: 4.5 (SEM, 0.11) versus 17.3 (SEM, 0.5) for normal and eczematous skin, respectively; p< 0.05]. A strong membranous CD99 immunoreactivity was observed in the epithelial cells (epidermis and adnexa) of both normal and eczematous skin. No CD30+ cells were observed in normal skin. Conclusions: This study demonstrates for the first time that cells expressing leukocyte CD30, CD57 and CD99 antigens are present in eczematous dermatitis. The high cell counts in eczematous dermatitis suggest a possible link between the capabilities of these cells and development of these lesions.
Keywords: Eczema, Normal skin, CD30, CD57, CD99
Cite this paper: Mahmoud R. Hussein, Hanan A. Assaf, Eman E. Abu-Dief, Amira A. Abdel-Motaleb, Asmaa Mahmoud Ahmed, Abeer Refaiy, Expression of CD30, CD57 and CD99 Leukocyte Antigens in Eczematous Dermatitis, American Journal of Dermatology and Venereology, Vol. 5 No. 3, 2016, pp. 51-57. doi: 10.5923/j.ajdv.20160503.03.
1. Introduction
- Eczematous dermatitis is an inflammatory condition characterized by the development of pruritic, erythematous, scaly and crusted eruptions on the extensor surfaces of the extremities. On histology, eczematous lesions are characterized by dermal inflammatory infiltrate which is usually associated with fibrosis [1]. The pathogenesis of eczematous dermatitis is poorly understood, but the alterations in the immune cells are possible underlying etiologic factor. Inflammation is the response of the vascularized living tissue to injury. It has two main components; a vascular wall response and an inflammatory cell response. Several immune cells and a number of leukocyte antigens such as CD30, CD57 and CD99 are involved in this process [2]. However, to date our knowledge about the expression pattern of these antigens in normal and eczematous skin is limited.CD30 is a protein belonging to the tumor necrosis factor receptor superfamily. Its expression is mostly restricted to virus-infected lymphocytes, neoplasms of lymphoid origin and a subset of activated T cells which produce Th2-type cytokines. The ligand for CD30 is present on activated T cells, resting B cells, granulocytes, and the medulla of the thymus (epithelial cells and Hassal's corpuscles). The biological functions of CD30 are pleiotropic and related to co-stimulatory signals. CD30 expression has been found in increasing numbers of cutaneous non-neoplastic inflammatory disorders [3, 4]. Natural killer (NK) cells comprise 10% to 15% of the peripheral blood mononuclear cells. They are characterized by their morphologic appearance (large granular lymphocytes) and phenotype (CD3-CD56+). They can be detected using immunoreactivity for CD56, CD16 and CD57 proteins. CD57 (Leu™-7) antigen is a human lymphocyte antigen, that is a carbohydrate structure associated with myelin-associated glycoprotein. The CD57 antigen is expressed on a subset of NK lymphocytes and a subset of T lymphocytes [5, 6]. NK cells are widely accepted to play a role in the pathogenesis of some inflammatory conditions. T cells expressing CD57 (a natural killer cell marker) increases under various conditions such as atopic eczema, infective dermatitis, psoriasis and lichen sclerosus et atrophicus [7, 8]. CD99 is a 32-kDa cell surface protein. It is a small highly O-glycosylated cell-surface molecule expressed on most leukocytes. CD99 is also found on endothelial cells, where it mediates transendothelial migration of human monocytes and lymphocyte recruitment into inflamed skin in the mouse [9, 10]. This delivery of leukocytes from the bloodstream across vascular endothelium to the site of inflammation is a critical function of inflammation. Dufour and his colleagues indicated that CD99 is expressed in leukocytes and at human endothelial cell contacts. Human CD99 is involved in homophilic interaction between the two cell types and participates in the transendothelial migration of monocytes and polymorphonuclear neutrophils in vitro [11]. Skin is an immunologically active organ and is considered to be a defense barrier against several antigens. It acts against them by recruiting and activating the lymphocytes and histiocytes, which are responsible for the immune response. Eczematous dermatitis is characterized by a dermal and epidermal infiltrate comprised predominantly of lymphocytes and histiocytes. However, studies assessing the status of CD30+, CD57+ NK and CD99+ cells in these lesions are lacking. In order to get information regarding the distribution of leukocyte antigens on the lymphoid cells of normal skin and that of eczematous dermatitis, immunohistochemical staining was performed using antibodies against CD30, CD57and CD99 antigens.
2. Materials and Methods
- Tissue specimens: The total number of specimens was 50 cases of eczematous dermatitis (untreated). Additional 10 cases (matched for age and sex and site) of normal skin served as a control group. The normal skin was obtained during course of plastic surgeries. Immunohistochemical studies: Immunostaining was carried out as previously described [7]. Immunohistochemical staining was performed for CD30, CD57 and CD99 on 4 microns thick, formalin fixed, paraffin wax embedded sections, using the streptavidin–biotin immunoperoxidase technique. After deparaffinization and rehydration, sections were placed in 3% hydrogen peroxide for 20 minutes to inactivate endogenous peroxidase, and treated by microwave at 121°C in citrate buffer (10 mM, pH 6.0) for 10 minutes as an antigen retrieval method. After cooling to room temperature for 30 minutes, non-specific protein binding was blocked with 10 min. exposure to 10% normal goat serum. Monoclonal antibodies were used. Monoclonal anti-CD57 antibody (Anti-CD57 monoclonal, Clone NCI; Catalogue # 1166; Immunotech, Marseillei, Cedexa, France, BP.177) was used (ready to use) for 30 minutes at room temperature [12]. CD57 was chosen, as it recognizes a carbohydrate antigen presents on NK cells (mononuclear cell with most non-MHC restricted ctytotoxicity). Monoclonal antibodies were also used for detection of CD99 and CD30 antigens {monoclonal anti-CD99 antibody (CD99, MIC2 gene product, Clone 12E7, Code M3601 and anti-CD30 antibody (Anti-Human CD30, clone Ber-H2, code Nr.M0751) DakoCytomation Denmark, DK-2600 Glostrup} at a dilution of 1:100. A secondary-staining system (LSAB2, DakoCytomation Denamark A/S-DK-2600 Glostrup) was used according to the manufacturer instructions. Sections were next treated with Peroxidase-labeled Streptavidin for 30 min. at room temperature and incubated with 14-diaminobenzidine and 0.06% H202 for 5 min. The sections were counterstained with hematoxylin, dehydrated in alcohol, cleared in xylene and cover-slipped. The immunostaining experiments were repeated three times to get consistent results [1, 13]. As a negative control for each case, the primary antibody was replaced with normal rabbit serum [1]. Specimens diagnosed as embryonal carcinoma (CD30), NK cell lymphomas (CD57) and Ewing’s sarcoma (CD99) were used as positive controls. Evaluation of CD30+, CD57+ NK and CD99+ cells: CD30 antibody variably produces cytoplasmic, membranous and Golgi staining in the activated B and T lymphocytes. CD57 antibody produces membranous staining in NK cells. Dark brown membrane staining indicated the presence of immunoreactivity for the CD99 cell-surface antigen. The sections were scanned at low magnifications to identify the most positive areas of the skin (hotspot areas). CD30+, CD57+ NK and CD99+ cells were counted in the dermis. For each biopsy, 10 fields were studied (X400). The counts of CD30, CD57 and CD99 positive cells were combined to give a final figure for each antigen. The positive cells were expressed as mean and standard error of mean (SEM) [1, 13]. Analysis of variance (ANOVA) and Student t- tests, with a statistical significance of p<0.05 were performed. Calculations were done with the statistical package SPSS for windows, version 10.0. Statistical significance was defined as p<0.05 [1].
3. Results
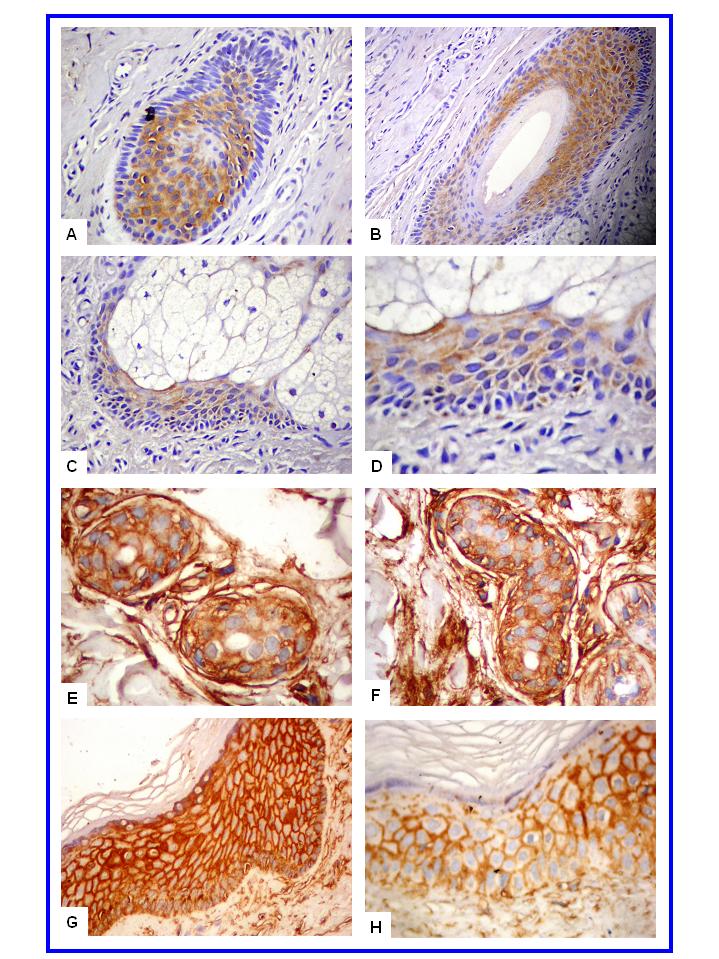
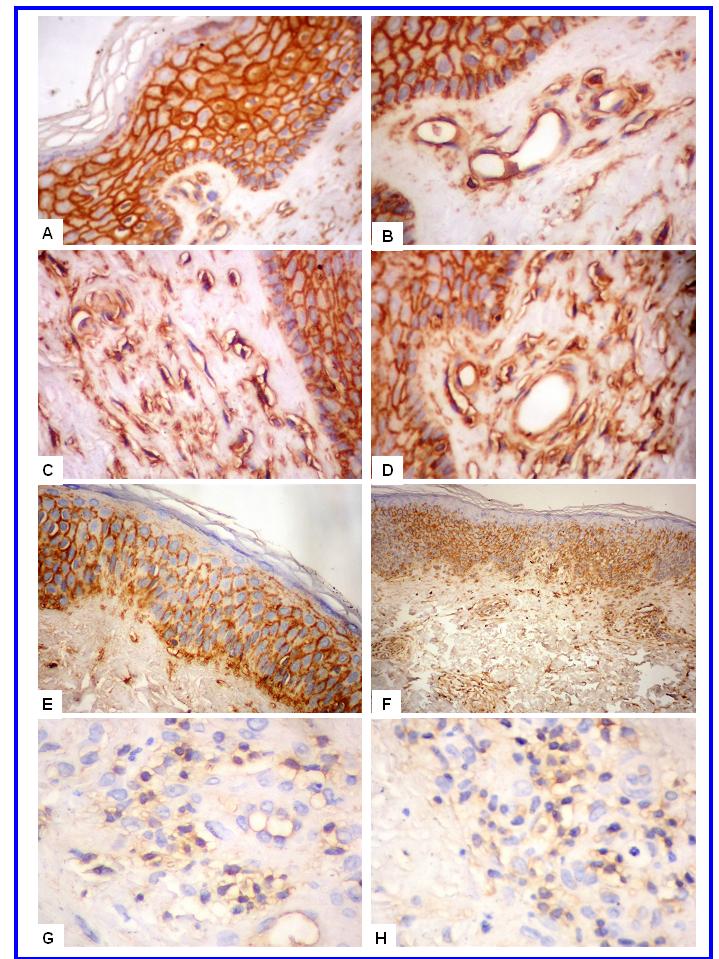
- In normal skin, CD57+ NK and CD99+ lymphocytes/ histiocytes were occasionally seen in perivascular location. No CD30+ cells were observed in normal skin. In eczematous dermatitis skin, CD30+, CD57+ NK and CD99+ lymphocytes/histiocytes cells were seen in the dermis (CD30, CD57 and CD99) and occasionally in the epidermis (CD57). CD30+, CD57+ NK and CD99+ cells were seen scattered in the upper part of the papillary dermis. Compared with normal skin, a statistically significant higher count (mm2) of these positive cells was seen in eczematous [CD30: 0.0 (SEM, 0.0) versus 0.23 (SEM, 0.0); CD57: 0.16 (SEM, 0.07) versus 1.52 (SEM, 0.10) and CD99: 4.5 (SEM, 0.11) versus 17.3 (SEM, 0.5) for normal and eczematous skin, respectively; p< 0.05]. A strong membranous CD99 immunoreactivity was observed in the epithelial (epidermis and adnexa) and endothelial cells of both normal and eczematous skin. A summary of these findings is presented in Figures 1-4 and in Table 1.
|
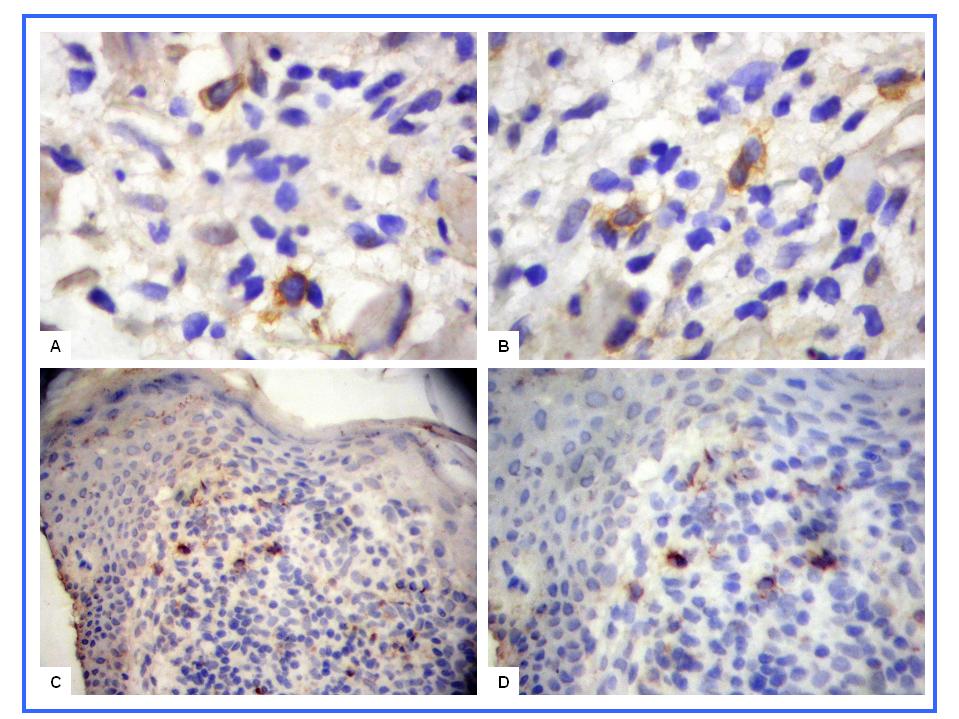 | Figure 1. CD57+ NK and CD30+ cells in eczematous dermatitis. CD30+ cells (A-B, X400) and CD57+ NK cells (C-D, X200) are seen in the papillary dermis |
4. Discussion
- To date, the expression pattern of CD30, CD57 and CD99 leukocyte antigens in eczematous dermatitis is largely unknown. This study examined this issue and also described for the first time the expression pattern of the stated molecules in normal skin. CD30 is a molecule expressed on the surface of lymphoid cells, including activated T lymphocytes. Its significance on T cells in physiologic and pathologic conditions is not yet completely clarified. However, the fact that CD30 appears to be expressed on the surface of T-cell clones producing Th2/Th0-type cytokines favored an increasing interest on the molecule [14]. The presence of occasional CD30+ cells in the dermal inflammatory infiltrate of eczematous dermatitis concurs with findings in other inflammatory skin lesions such as allergic contact dermatitis [15]. The scarcity of CD30 + cells in the lesional skin of eczematous dermatitis suggests that a Th0/Th2-type response does not predominate in the skin of these lesions. Caproni et al examined CD30 expression in the skin biopsies of patients with active atopic dermatitis and allergic contact (nickel-induced) dermatitis. Remarkable numbers of CD30+ cells were seen in atopic dermatitis. In contrast, virtually no CD30+ cells were found in the skin of patients with allergic contact dermatitis [16].Expansion of CD57+lymphocytes is associated with infections and autoimmune diseases and may be the result of chronic excessive antigen challenge. In these pathologic states, CD57+ NK cells participate in the suppression of cytolytic activity to limit tissue damage [17]. The finding of CD57+ NK cells in eczematous dermatitis is in agreement with previous studies in psoriatic skin, infective dermatitis, lichen sclerosus et atrophicus and the inflammatory stage of morphea [7, 8, 17]. It also suggests a possible role for NK cells in the development of eczema. Cameron and his colleagues examined CD57+ NK cells in normal and lesional skin (from the active edge of a psoriatic plaque and from uninvolved skin) and they found increased density of NK cells in these lesions both in the epidermis and in the dermal infiltrate [7]. Similarly, Bittencourt et al examined the distribution of CD57+ cells in infective dermatitis associated with HTLV-I. The infiltrate consisted predominantly of CD57+ cells in the dermis and epidermis [8]. Gober et al. examined the distribution of NK cells in the cellular infiltrate of allergic contact dermatitis. NK cells were found in all the skin biopsy specimens studied, ranging from 1.72 to 33% of the cellular infiltrate. These NKT cells were activated in all cases. They expressed cytokine transcripts for IFN-gamma and IL-4 [18].CD99 is an interesting adhesion molecule. It is a sialomucin-type glycoprotein. The presence of strong CD99 protein reactivity in the epidermis and adnexal structure of the normal and eczematous dermatitis skin is consistent with its ubiquitous expression, albeit to varying degrees, by different normal and abnormal cell types [19, 20, 21]. The strong CD99 membranous staining (keratinocytes and adnexal epithelium) revealed by this study is consistent with its involvement in cell-cell adhesion [11] and maintenance of cellular morphology [22]. In this study, normal skin showed few dermal CD99+ histiocytes and lymphocytes. Conversely, skin from eczematous dermatitis showed higher counts of CD99 + cells .These findings are consistent with CD99 role in the inflammatory process. CD99 can regulate the adhesion and diapedesis of leukocytes to inflamed vascular endothelium [19, 23, 24]. The strong CD99 + reactivity of the endothelial cells of the dermal blood vessels (eczematous dermatitis) further suggest its active role in these lesions [25]. Bixel et al cloned a mouse cDNA coding for a protein 45% identical in its sequence with human CD99. They generated CD99 antibodies against this mouse homolog that were able to detect the antigen on most leukocytes and on endothelia of various tissues. CD99 antibodies inhibited transendothelial migration of lymphocytes in vitro. They also inhibited the recruitment of in vivo-activated T cells into inflamed skin as well as edema formation in a cutaneous delayed-type hypersensitivity reaction. Taken together, these findings indicate that CD99 can participate in the transendothelial migration of lymphocytes and in their recruitment to inflamed skin in vivo. They also raise the possibility that CD99 may be a useful therapeutic target for interference with cutaneous inflammatory processes [9].To conclude, this study showed for the first time the expression of some leukocyte antigens (CD30, CD57 and CD99) in the normal skin and that of eczematous dermatitis. The findings reported here suggest that CD30+, CD57+ and CD99+ cells are components of the chronic microenvironmental changes associated with the development of eczematous dermatitis. The presence of these molecules may be markers of skin inflammation and potentially, disease chronicity. The exact roles of the stated molecules in these lesions are still unclear, although they may modulate inflammation and act as a source of Th (1) cytokines (CD57). Further detailed studies directed to determine the role of these antigens in chronic dermatitis would help to understand the role of these molecules in inflammatory events.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML