-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2016; 5(2): 25-33
doi:10.5923/j.ajdv.20160502.02

Safety and Efficacy of Tinefcon (Sphaeranthus indicus) Cream in Treatment of Plaque Psoriasis – A Phase II B, Double Blind, Randomized, and Placebo Controlled Study
Chitra Nayak 1, Vishalakshi Viswanath 2, Rachita Dhurat 3, Rajesh Jadhav 4, Anirudh Tripathi 5, Anshuman Telang 6, S. N. Dixit 7, Mukund Shrivastav 8, Sudhakar Grandhi 9, Aasin Maurya 10, Somesh Sharma 11, Vijaysingh Chauhan 12, Ashish C. Suthar 12
1Dept of Dermatology, BYL Nair Hospital and TN Medical College, Mumbai, India
2Dept of Dermatology, Chhatrapati Shivaji Maharaj Hospital and Rajiv Gandhi Medical College, Thane, India
3Skin and VD Department (OPD 16), College Building, LTMM & LTMG Hospital Sion, Mumbai, India
4Life Care Polyclinic, Vartak Nagar, Thane (W), India
5Life Veda, Worli, Mumbai, India
6Dr. Telang Skin Hospital Birdopur, Mahmurganj, Varanasi, India
7Skin Centre, Beniya Bagh, Varanasi, India
8SSPG Hospital, Varanasi, India
9Pentagon Research Pvt Ltd, Aundh, Pune, India
10Opulence, Santacruz East, Mumbai, India
11Piramal Enterprises Limited, Goregaon-East, Mumbai, India
12Phytomedicines Department, Piramal Enterprises Limited, Goregaon-East, Mumbai, India
Correspondence to: Ashish C. Suthar , 2Phytomedicines Department, Piramal Enterprises Limited, Goregaon-East, Mumbai, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Sphaeranthus indicus is acknowledged for its immunomodulatory and anti-inflammatory activities, and possess anti-TNF (tumour necrosis factors) activity. Objective: To evaluate the safety and efficacy of Tinefcon (Sphaeranthus indicus) cream in treatment of plaque psoriasis. Method: This was a phase-IIB, double-blind, randomized, and placebo controlled clinical trial. Total 86 patients diagnosed with stable plaque psoriasis were randomized (2:1 ratio) into two groups and studied for a period of 90 days. Medications were randomly assigned into 58 subjects from Tinefcon cream group and 28 subjects from placebo group. The efficacy parameters studied were mean percent change in local psoriasis severity index (LPSI) of a target lesion, dermatology quality of life index (DQLI), visual analogue scale (VAS), physicians’ global assessment (PHGA), and patient’s global assessment (PTGA). The cosmetic acceptance and safety profile of Tinefcon cream was also evaluated. Results: Tinefcon cream reduced the target lesion severity by 69.94% as compared to 51.11% reduction (t = 3.0, p = 0.004) in placebo. 70.7% patients achieved reduction ≥ 50% as compared to 42.9% in placebo group (F = 0.013, p = 0.013) and 41.4% patients achieved reduction ≥ 75% as compared to 21.4% in placebo group (F = 0.06, p = 0.06) in target lesion severity. The mean percentage reduction of itching and pain assessed by DQLI in Tinefcon cream was 62.89% as compared to 43.45% in placebo and VAS produced significant mean percent reduction of target lesion by 68.35% in Tinefcon-group as compared to 53.50% in placebo group. In addition, no adverse or serious adverse events occurred during the study. Conclusions: Tinefcon cream was safe and effective in the treatment of stable plaque psoriasis.
Keywords: Plaque psoriasis, LPSI, Tinefcon cream, Sphaeranthus indicus
Cite this paper: Chitra Nayak , Vishalakshi Viswanath , Rachita Dhurat , Rajesh Jadhav , Anirudh Tripathi , Anshuman Telang , S. N. Dixit , Mukund Shrivastav , Sudhakar Grandhi , Aasin Maurya , Somesh Sharma , Vijaysingh Chauhan , Ashish C. Suthar , Safety and Efficacy of Tinefcon (Sphaeranthus indicus) Cream in Treatment of Plaque Psoriasis – A Phase II B, Double Blind, Randomized, and Placebo Controlled Study, American Journal of Dermatology and Venereology, Vol. 5 No. 2, 2016, pp. 25-33. doi: 10.5923/j.ajdv.20160502.02.
Article Outline
1. Introduction
- Plaque psoriasis is one of the most common types of psoriasis. It is a complex autoimmune inflammatory skin disorder clinically characterized by the presence of raised thickened patches of red skin covered with silvery micaceous scale. The skin lesions are variably pruritic, and even lesions are developed in traumatized areas. The lesions can commence as erythematous macules or papules, external to peripheral, and amalgamate to form plaques of one or more than one centimetre in diameter. The plaques may gradually extend into different configurations such as psoriasis gyrata (curved linear), annual psoriasis (ring like) or psoriasis follicularis (minute scaly papules are present at openings of pilosebaceous follicules) [1]. The stable and slowly enlarged papules which remain unchanged for longer period of time make it distinguishable from other types of psoriasis. It commonly affects elbows, knees; gluteal cleft, and scalp [2]. Guttate, pustular, and nail psoriasis are the other major clinical subtypes of psoriasis [1].No prevalence information is available for plaque psoriasis. However, according to world health organization 2% peoples are affected with psoriasis worldwide, but the prevalence is increased further in developed countries i.e. 4.6%. Additionally, approximately two third of populations have been afflicted with a mild form of psoriasis [3]. According to National Institute of health, as many as 7.5 million Americans (approximately 2.2%) have been afflicted with psoriasis; some studies also confirmed that between 10 to 30% of psoriasis peoples also develop psoriatic arthritis. The prevalence of psoriasis in African Americans is 1.3% compared to 2.5% Caucasians [4]. In India, 1-3% prevalence of psoriasis is found whereas 0.3-1% patients are present with psoriatic arthritis [5]. The assessment of psoriasis includes measurement of symptoms and involvement such as body surface area (BSA), psoriasis area and severity index (PASI), PHGA as well as quality of life measures such as dermatology DQLI, or short form (SF-36) health survey. On basis of BSA and PASI, psoriasis is classified into mild, moderate, and severe categories. For instance, mild psoriasis: ≤ 5% BSA involvement, moderate psoriasis: PASI ≥ 8, severe psoriasis: the rule of ten, PASI ≥ 10 or DQLI ≥ 10 or BSI ≥ 10%. The major comorbidities associated with psoriasis are depression, hypertension, obesity, diabetes mellitus, Crohn’s disease, and anxiety etc. [6]. Psoriasis is orchestrated by various cytokines and chemokines. Pro-inflammatory cells are considered to be responsible for many of the histopathological changes seen in psoriasis patients, for example, interferon-γ and TNF-α may stimulate the expression of major histocompatibility complex (MHC)-II molecules and intracellular adhesion molecule 1 (ICAM-1). Angiogenesis could be stimulated by vascular endothelial growth factors and TNF-α. Simultaneously, interleukin (IL)-1 triggers mast cells, granulocyte macrophage-colony stimulating factors (GM-CSF) activates neutrophils, nerve growth factor (NGF) arouses the growth of cutaneous nerves, and keratinocyte proliferation is promoted by IL-6 and transforming growth factors, these all with chemokines leads to psoriasis eventually. In particular, TNF-α affects the functions of different cell type in psoriatic skin and inhibition of pro-inflammatory cytokines have emerged as new pathogenesis oriented treatment [7, 8]. The 7-hydroxy frullanolide fraction from Sphaeranthus indicus exhibited anti-TNF activity in in vitro and in vivo and thus it can be one of the possible treatment options for plaque psoriasis [9]. Generally topical treatment includes emollients, keratolytics, coal tars, anthralins corticosteroids, vitamin D3 analogues, and tazarotene etc. Among them topical corticosteroids is one of the most common prescribed treatments, but is associated with various adverse effects, like on longer use it loses its effectiveness (tachyphylaxis) and causes atrophy of skin. Mild irritant contact dermatitis, burning, pruritus, oedema, peeling, dryness and erythema of skin are the side effects associated with Vitamin D3. One of the vitamin D3 analogue (calcipotriol) and anthralin has been labelled with pregnancy category C, while tazarotene a retinoid compound is labelled with pregnancy category X and avoided in women of childbearing capacity unless effective contraception is being used [6]. Therefore need arises for newer topical treatment that can effectively improve the clinical sign and symptoms; and can be applied for prolong period of time without any tachyphylaxis or adverse effects. Hence, Sphaeranthus indicus with anti-TNF activity [9] and a well-known Indian herb has been explored for its immunomodulatory, antioxidant effect, mast cell stabilizing action, hepatoprotective effect, wound healing, and anti-inflammatory actions [10], and formulated in cream for the treatment of plaque psoriasis. The cream was made from 20% extract of Sphaeranthus indicus, evaluated in the treatment of mild to moderate plaque psoriasis as compared to placebo (aminoglycan) cream. This study was conducted at a national level across nine locations approved by respective institutional ethics committees in India.
2. Methods
2.1. Preparation of Investigational Product
- The investigational product Tinefcon was the topical cream for external application consisting of extract prepared from flowers and fruit heads of Sphaeranthus indicus. The cream contained 20% extract of Sphaeranthus indicus.
2.2. Study Population
- The subjects with stable plaque psoriasis for at least 6 months with 2% to 10% affected BSA, target plaque area of 16cm2, and LPSI score ≥ 5 excluding palms, soles, face, scalp, groin, axillae or other intertriginous areas were included in the study. Moreover, subjects devoid from cardiac, pulmonary, other dermatological conditions, gastrointestinal, neurological, or psychiatric diseases were recruited for the study. The subjects who had received systemic therapies of methotrexate or cyclosporine in past three months were excluded from the study. Likewise, subjects who had received TNF-α, phototherapy - ultraviolet B, psoralen with ultraviolet A (PUVA), lithium, β-blockers, antimalarial, and non-steroidal anti-inflammatory drugs were also excluded. Subjects who received Ayurvedic, Homeopathic or Herbal drugs, medicated oil or creams for one month, three months prior to screening visit had been excluded. Additionally, subjects receiving topical treatments such as tars, salicylic, dihydroxy anthrol, and vitamin D analogues, two weeks prior to randomization were also omitted from the study. Written informed consent was obtained prior to the subject’s entering the study. This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonization (ICH) Good Clinical Practice (GCP) Guidelines.
2.3. Study Design and Disposition
- This study was a phase II B, randomized, double blind, placebo controlled, and multicentre study. Total 107 patients were enrolled on the basis of selection criteria, of which 86 patients completed the study. The subjects were randomly allocated in 2:1 ratio, in which Tinefcon cream (group A) was applied in 58 subjects, while placebo (aminoglycan) cream (group B) was in 28 patients. All the evaluated patients underwent an informed consent process followed by screening procedure within 3 days of participation. The patients were instructed to apply Tinefcon cream or placebo cream on affected area on the entire body twice a day for 3 months. The study was conducted for the period of 14 weeks including screening period. The efficacy of Tinefcon cream was evaluated on 30th day (visit 1), 60th day (visit 2), and 90th day (visit 3). In addition, safety parameters were also evaluated on the day of screening and 90th day. All the products were dispensed in 200 gm PET bottle, and drug accountability was checked by recording in case record form for remaining or unused quantity of cream in terms of percentage of the total unused medication.
2.4. Assessment
- The efficacy parameters like LPSI score, VAS for itching, PHGA, PTGA, Question 1 of DQLI, Question 10 of DQLI, and DQLI were assessed on every follow up visit. In primary efficacy end point, the obtained results were represented as mean ± SD for comparison of variables like scales, erythema, induration, and total LPSI. The values obtained on visit 1, 2, and 3 were compared with their respective values obtained at baseline. Moreover, % reduction (as compared to baseline) was also calculated. Similarly, the secondary end points such as VAS, DQLI, Question 1 of DQLI, Question 10 of DQLI PHGA, and PTGA were also assessed. The cosmetic acceptance of therapy was also performed on visit 3. The safety parameters like complete blood count, renal and liver profile, routine urine and microscopy were measured on the screening day and 90th day.
2.5. Statistical Analysis
- Data was analysed using SPSS V 10.0 (Statistical Package for the Social Sciences, Version 10.0) package. Data was given as Mean ± SD or Frequency (Percentage) as per the type of data. Chi square tests (with Yate’s Correction) and Fisher Exact Probability Tests were applied to compare percentages between 2 groups. Student’s paired t tests were applied to compare means of related (before - after) data. Student’s unpaired t tests were applied to compare means of unrelated data. Friedman Chi square tests (Non parametric ANOVA) were applied to compare overall change of the same patients across various time points. Statistical Analysis was carried out on the completed patients. Level of significance was considered as p > 0.05.
3. Results
3.1. Patient Demographic and Baseline Characteristics
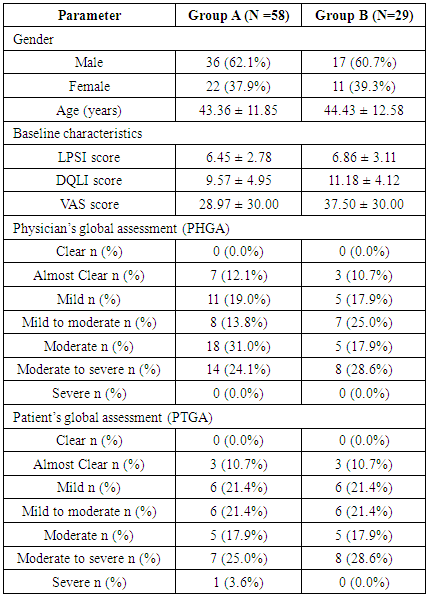
- The demographic data was evaluated on the basis of per protocol population. Patients demographic, baseline characteristics, PHGA, and PTGA were very similar between both the groups (Table 1).
|
3.2. Primary Efficacy Assessment
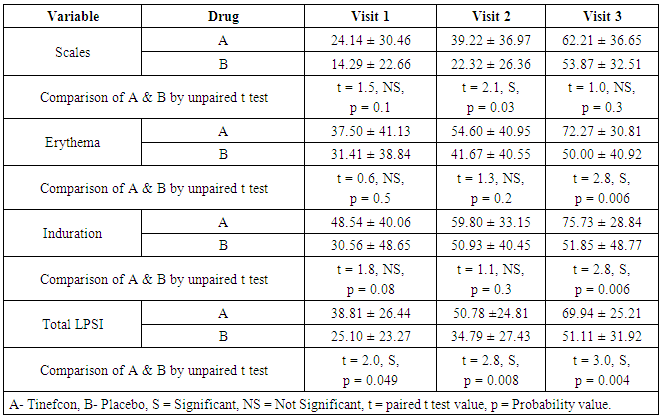
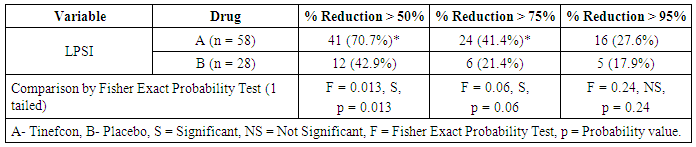
- The comparison of percent reduction (as compared to baseline) in primary efficacy end point variables like scales, erythema, induration, and total LPSI were evaluated by using unpaired t test in between group A and group B (Table 2). The data was represented as mean ± SD (% reduction values). On visit 3, the mean percent reduction of erythema was significantly higher in group A (72.27 ± 30.81) than group B (50.00 ± 40.92) (t = 2.8, p = 0.006) mentioned in Figure 1a. Similarly mean percent reduction of induration was significantly higher in group A than group B (75.73 ± 28.84 vs. 51.85 ± 48.77) (t = 2, p = 0.006) (Figure 1b). In addition, the mean percent of total LPSI was significantly higher on every visit in group A compared to group B. The total LPSI score on visit 1, 2, and 3 in group A and group B was 38.81 ± 26.44 vs. 25.10 ± 23.27 (t = 2, p = 0.049), 50.78 ± 24.81 vs. 34.79 ± 27.43 (t = 2, p = 0.008), and 69.94 ± 25.21 vs. 51.11 ± 31.92 (t = 3, p = 0.004) respectively (Figure 1c).
|
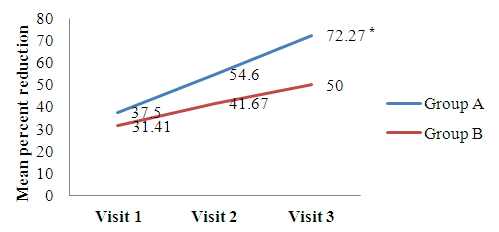 | Figure 1a. Mean percent reduction of erythema score compared with baseline. [Group A (Tinefcon) vs. Group B (placebo)] |
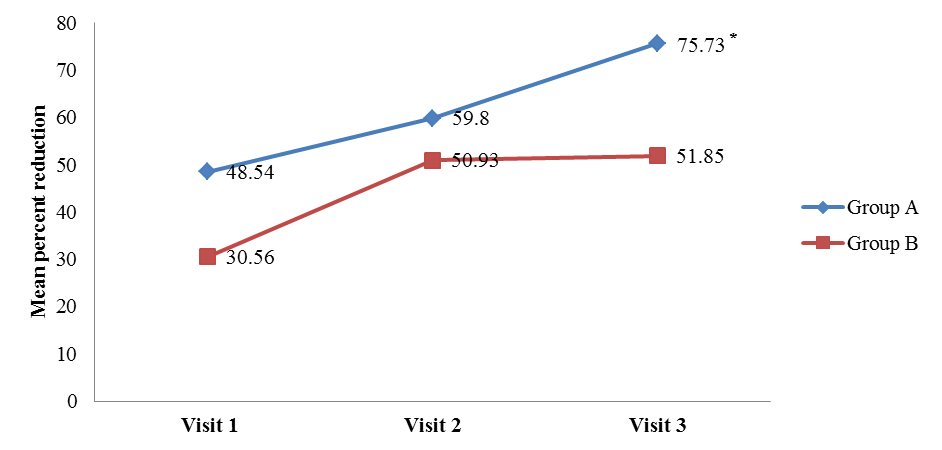 | Figure 1b. Mean percent reduction of induration compared with baseline. [Group A (Tinefcon) vs. Group B (placebo)] |
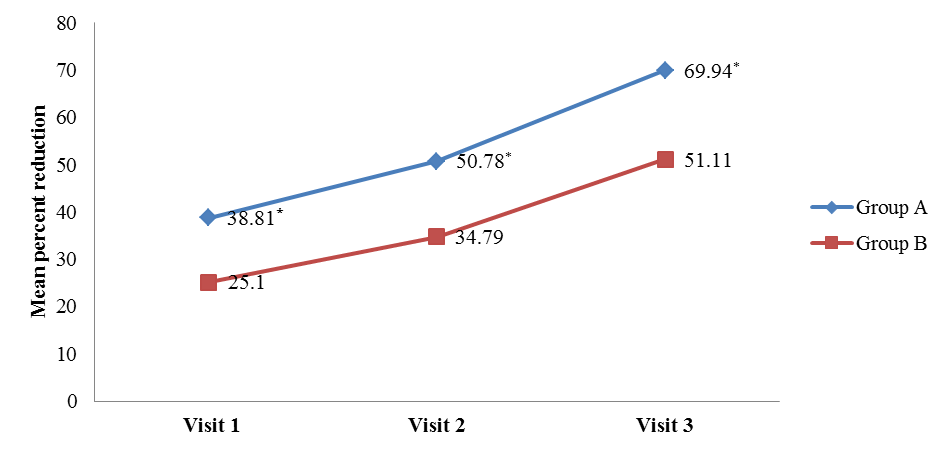 | Figure 1c. Mean percent reduction of total LPSI compared with baseline. [Group A (Tinefcon) vs. Group B (placebo)] |
|
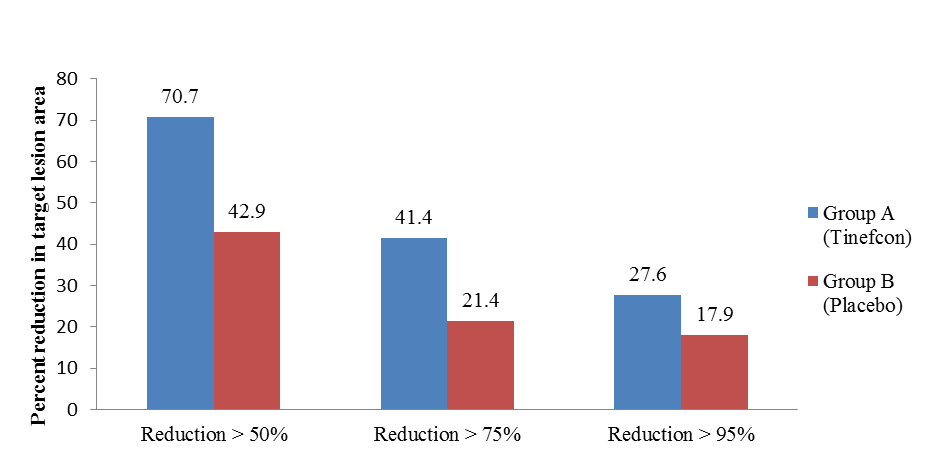 | Figure 2. Percent reduction in target lesion area according to LPSI score |
3.3. Additional Efficacy Assessment
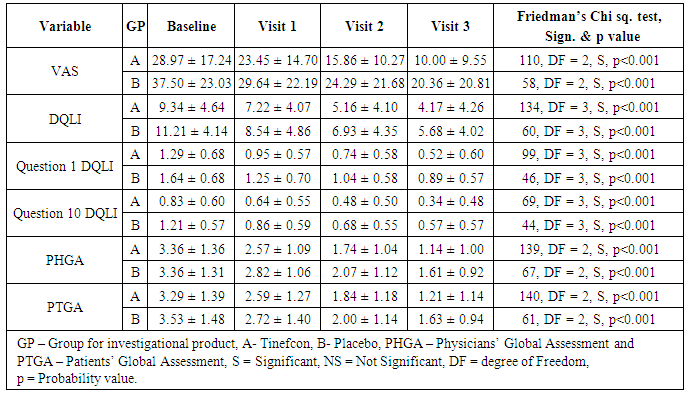
- The mean VAS, DQLI, Question 1 DQLI, Question 10 DQLI, PHGA, and PTGA in group A and group B were compared with their respective baseline values obtained on visits 1, 2, and 3. The data was represented as mean ± SD and statistically analysed by using Friedman’s Chi square (nonparametric ANOVA). Comparison of mean percent reduction in all secondary efficacy parameters was also evaluated as conducted in primary efficacy end point. The data was represented as mean ± SD (% reduction values). All the parameters included in secondary efficacy points had been reduced significantly in a proportional manner compared to their baseline values (Table 4), but no statistical significant difference had been noticed in between group A and group B (Table 4). Only Question 1 DQLI and VAS on visit 3 showed statistically higher percent reduction in group A compared to group B. The mean percent reduction of itching and pain assessed in Question 1 DQLI in group A was 62.89 ± 42.57 as compared to 43.45± 38.31 (t = 2.0, p = 0.047) (Figure 3a) and VAS showed significant reduction (t = 2.2, p = 0.03) in mean percentage score of target lesion by 68.35 ± 27.40 in group A as compared to 53.50 ± 33.70 in group B (Figure 3b).
|
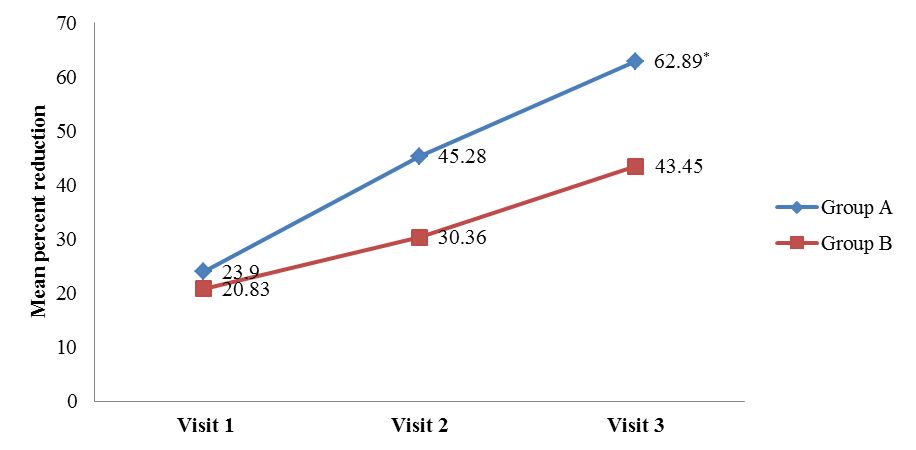 | Figure 3a. Mean reduction of Question 1 DQLI compared with placebo [Group A (Tinefcon) vs. Group B (placebo)] |
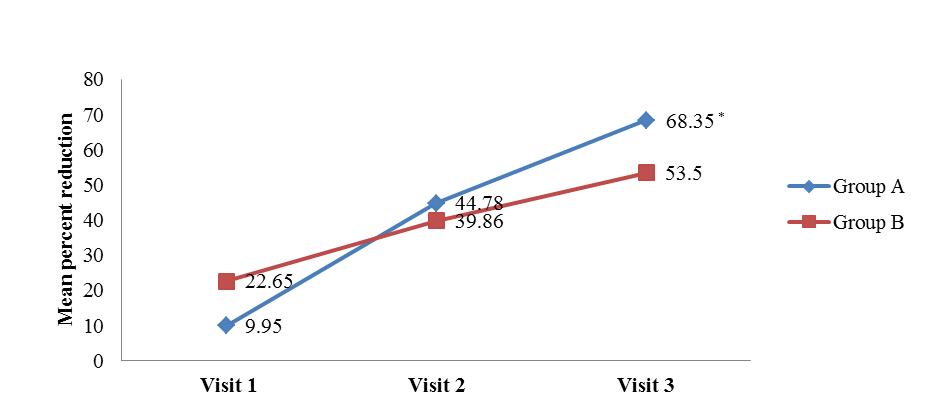 | Figure 3b. Mean reduction of VAS compared with placebo [Group A (Tinefcon) vs. Group B (placebo)] |
3.4. Physicians and Subjects Global Assessment
- Physicians’ global assessment (PHGA)The PHGA categorized psoriasis on the basis of severity. In this assessment patients were graded in to the categories of clear, almost clear, mild, mild to moderate, moderate, moderate to severe and severe condition. The improvement in psoriasis was assessed by comparing baseline severity to severity condition at visit 3. The PHGA clearly proves the efficacy of Tinefcon cream in treatment of psoriasis, as in group A the psoriasis was cleared in 29.3% of patients, while in Group B psoriasis was cleared in 10.7% of patients. In Group A the condition of mild to moderate psoriasis was decreased from 13.8% patients to 3.4% patients. While in group B, mild to moderate psoriasis was decreased from 25% to 17.9% of patients (Figure 4).
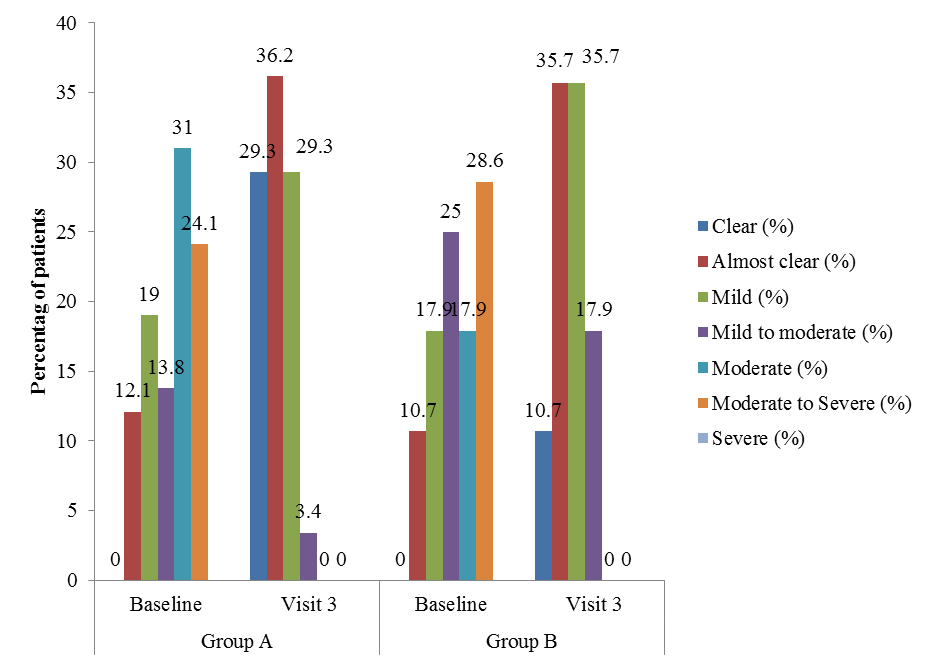 | Figure 4. Percent improvement in PHGA comparing visit 3 with baseline. [Group A (Tinefcon) vs. Group B (placebo)] |
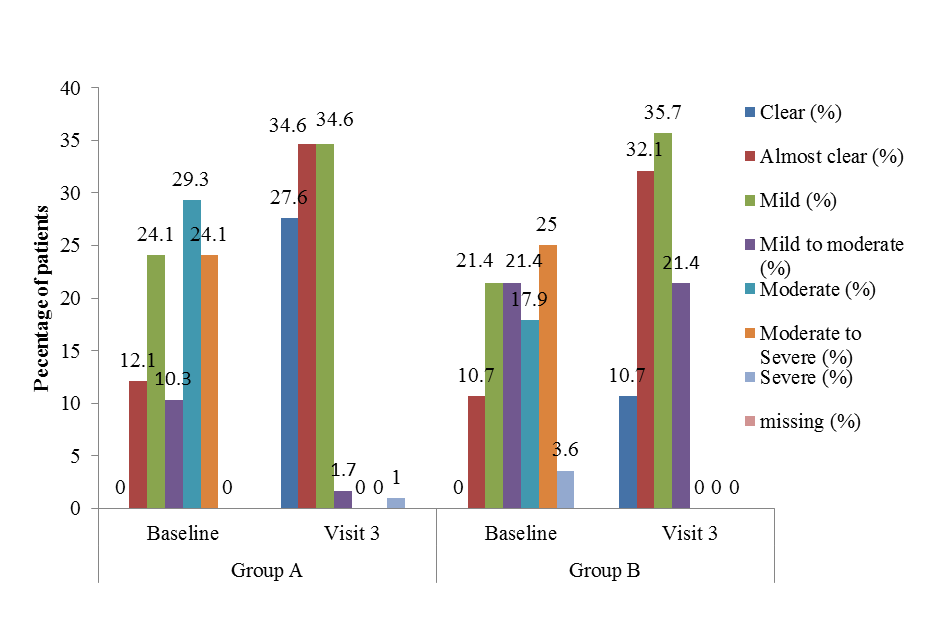 | Figure 5. Percent improvement in subjects’ global assessment comparing visit 3 with baseline [Group A (Tinefcon) vs. Group B (placebo)] |
3.5. Cosmetic Acceptance
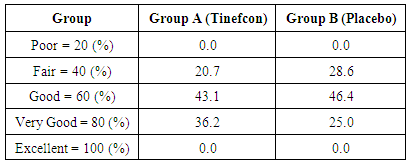
- Cosmetic acceptance of therapy was performed on visit 3 as secondary outcome, based on appearance, odour, oiliness, and adhesiveness. Only appearance was found better than the placebo cream. Total 36.2% patients from group A accepted the appearance was very good compared to 25.0% of patients from placebo group given in Table 5.
|
3.6. Safety
- Safety assessment during study included the monitoring of adverse events and vital signs as well as the clinical laboratory evaluations. Of 107 patients, 86 patients completed the entire study and none of them showed any adverse events or serious adverse events.
3.7. Adverse Events
- The causality of AEs was categorized as unrelated, unlikely, possible, and definitive. None of the patients from both the groups experienced AEs that were possible, probable and definitive to their respective treatments, except pustules that were considered as possible AEs related to treatment in Group A. Of 107 patients, 21 patients failed to complete the study. In which, 14 patients (5 patients = lost to follow up, 1 patient = failed to meet inclusion criteria, 1 patient = SAE, and 7 patients = AEs) were from Group A and 7 patients (4 patients = lost to follow up, 3 patients = AEs) from group B. Out of 14 patients, 7 patients were withdrawn due to adverse events (AEs) and 1 patient from serious adverse event (SAE). In SAE case, a 51 years old black male patient developed multiple raised lesions associated with burning sensation over the upper limbs, thighs, back, and abdomen associated with fever and chills. The oozing from existing psoriatic plaque was considered as SAE. The SAE occurred 11 days after first dose of Tinefcon. The patients were treated with prednisolone tablet (40 mg) O.D and emollients, but no information available about the resolution of AEs in both the groups. In group B, 3 patients were excluded from study due to occurrence of AE.
4. Discussion
- This was the first study, which determined the safety and efficacy of Sphaeranthus indicus cream in a randomized, double blind, placebo controlled, and multicentre study for the treatment of mild to moderate plaque psoriasis. Arguably, anti-TNF becomes the first-line agent in the treatment of plaque psoriasis as it is considered as a key mediator in the disease progression [11]. Sphaeranthus indicus also possesses anti-TNF activity, which made it one of the possible treatment options [9]. Currently, three anti-TNF agents’ viz. etanercept, infliximab, and adalimumab have been approved by the USFDA. Though the long term is being accumulated, but the available data proves its sustained effects [12]. This disease lasts for prolonged period of time; hence sustained effects of anti-TNF agents would be considered being conducive in the treatment and management of plaque psoriasis.LPSI system was used as primary efficacy end point in this study, which is the summation of scales, erythema, and induration. These three efficacy points are being utilized in several psoriasis studies. We applied Tinefcon cream or placebo cream on affected area for 3 months. In a study of Lin YN et.al (2007), chronic psoriatic patients were treated topically with Indigo naturalis for 8 weeks. They reported median score for scaling, erythema, and induration in Indigo naturalis group compared with the vehicle controlled group. Instead of the median score, we calculated mean percent reduction of these scale with their respective baseline values [13]. In another study, Lin YK et.al (2008) evaluated the efficacy and safety of Indigo naturalis in treatment of recalcitrant psoriasis for 12 weeks. They also adopted similar scales for evaluation [14]. In both these studies, reductions were observed for all three scales. Compared to their studies, we obtained significant mean percent reduction in erythema and induration. LPSI system was used by Ortonne JP et. al in their study, where they evaluated the efficacy of 0.3% tacrolimus gel and 0.5% tacrolimus cream comparing with calcipotriol ointment in mild to moderate plaque psoriasis patients. They measured the median percentage change in LPSI of the target lesion between baseline and week 12, but obtained no statistical difference between the groups [15]. On the contrary, we measured mean percent reduction and obtained significant difference between both the groups. The reason behind getting significant difference was assigning of placebo treatment to one group, whereas in Ortonne JP all the three involved treatment based anti-psoriatic activity [16, 17].We used DQLI, VAS, and Cosmetic acceptance as secondary efficacy end points. In past, these points were also used in assessment of psoriasis. Lu CJ et.al used DQLI and VAS to explore the therapeutic effects of auricular therapy combined with optimized Yinxieling formula on 84 psoriasis vulgaris outpatients. The DQLI score was decreased in both the groups, but no statistical significant difference had been occurred between them, similar to our study. Additionally, they had not found any statistical difference for VAS in between both the groups, whereas statistically higher percent reduction has been observed in our study [18]. Appearance of our cream was found better than placebo cream; the odour in Tinefcon group may be from the aromatic odour of Sphaeranthus indicus [10].Some common incidences such as pruritus, skin burning and erythema were reported in both the groups. SAE has occurred in one of the patient from the Group A coded as exacerbation of psoriasis, however, no possible, probable, and definitive correlation of Tinefcon cream existed with these AEs. Scope: The efficacy and safety of Tinefcon cream needs to be tested in bigger population size and for longer duration, so that its efficacy and safety can be further ascertained.
5. Conclusions
- On the basis of obtained results, we can conclude that Tinefcon cream has the ability to treat mild to moderate plaque psoriasis. The transdermal absorption of Sphaeranthus indicus extract may act as anti-inflammatory and immunomodulatory agent; preventing keratinocyte proliferation and subsequently skin thickness. The wound healing properties may restore the skin texture and reduce scar formation. Application of Tinefcon cream for 90 days was safe due to occurrence of minimal adverse events during the trial period. Hence, Tinefcon cream can be included in the list of available and possible management options for mild to moderate plaque psoriasis in adults.
ACKNOWLEDGEMENTS
- The authors are thankful to Dr. G. Tyebkhan for her expert comments and review of the manuscript, K. Salkar for administrative support and Karmic Lifesciences LLP for drafting of the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML