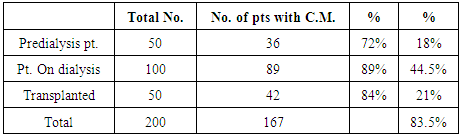
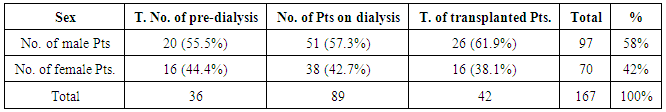
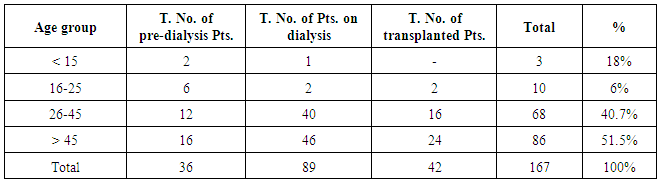
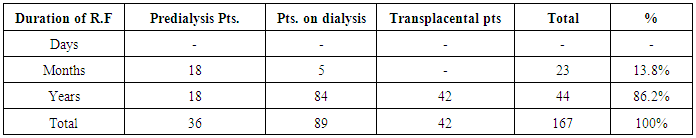
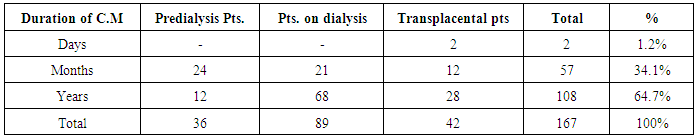
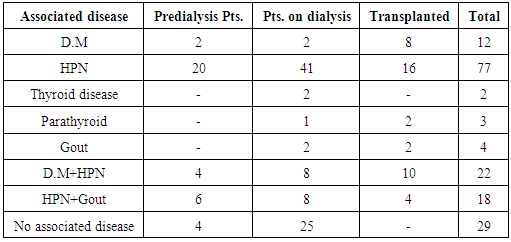
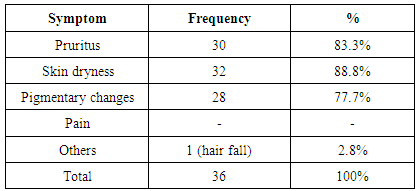
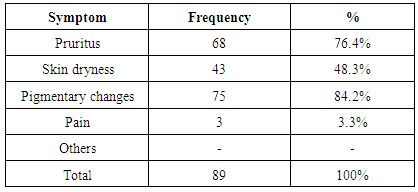
| [1] | Anderson S, and Brenner BM. Progessive renal disease: A disorder of adaptation :QJ Med 1989; 70:185, 1989. |
| [2] | Meguid EL Nahas. Progression of chronic renal failure in: Richard Johnes and John Feehly text book of comperhension clinical nephrology (first edition) 2001; 14:67.1. |
| [3] | Gilchrest BA et al. Clinical and histological cutaneous findings in uremia: Evedence from a dialysis - resistant, transplant – responsive micro angiopithy. Lancet 1980; 2: 1271. |
| [4] | Joseph L, Jorizzo and Elizabith F. Shevertz. Cutaneous changes related to chronic renal failure in Fitz Patrick text book of dermatology in general medicine 2000; 165 : 2058. |
| [5] | Maxwell MH et al. Clinical disorders of fluid and electrolyte metabolism, 4th ed 1987; New York, Mc Graw – Hill. |
| [6] | Brezis M et al. Acute renal failure, in the kidney, 4th ed, BM Brenner, FC Rector Jr (eds). Philadelphia, Saunders 1991; 993. |
| [7] | Brenner BM, Lazarus JM (eds). Acute Renal Failure, 3rd ed 1993; New York, churchil Living stone. |
| [8] | Glassock RJ. Nutrition, immunlogy and renal disease. Kidney Int 1983; 24: S 194. |
| [9] | Dewaurdner, H.E. The kidney. An outline of normal and abnormal structure and function 1973; P: 432. |
| [10] | Brickman A.S. P.H, Massvy S.G and Norman A.W. Impaired calcium absorption in uremic man, J. Lab .clin. Med 1982; 48 ,791. |
| [11] | Clarkson E.M, Eastwad J.B., koutsaimanis K.G and Dewaurdener H.E.. Net intestinal absorption of calcium – patient with CRF Kidney Int 1973; 3:258. |
| [12] | Parker R.F, Vergne – Marini P, Hull A.R, and pake C.Y. Jejenal absorption and secretion of calcium in patients with CRF on haemodialysis. J. Clin . investigation 1974; 54:359 [quoted from Andreucci , V.E.1982]. |
| [13] | Avioli L.V. Renal osteodystrophy and v.t.D . pt . on dialysis transplanted patient 1978; 7:244. |
| [14] | Atkius D. (1976). A possible role of 24,25 (oh)2 1)3 in bone resorption. Journal Eudocrinol, 69:28. |
| [15] | Stern P. Bone resorbing of 25 – oh stereoisomens of 24 – oh at D3 and 24, 25 (oh)2 D3. Bio.chem. Biophys. Res common 1975; 67, 965. |
| [16] | Lee S.W., Russell J., and Avioli L.V. 25 – oh 1,3 to 1,25 oh2 1,3: conversion impaired by systemic metabolic acidosis. Science 1977; 194, 994. |
| [17] | Lumb G.A, Mawer and Stanbury S.W. The apparent vitamin D resistance of CRF. Am . J . Med 1971; 50, 421. |
| [18] | Kanis, J.A, Oliver D., Ledingham J.G.G, and Russel R.G.G. Evidence that endogenous calcitorin protects against renal bone disease . Lancet 1976; 2, 1322. |
| [19] | Tranzer F.S, and Naria J.M. Calcitonin inhibition of intestinal phosphate absorption. Nature, london, 1973; 242, 221. |
| [20] | Haussler M.R., and Mc cain T.H. Basic and clinical concepts related to vitamin D metabolism and action N.E ng .J. Med 1977; 297, 974. |
| [21] | Habener J.F. and Potts J.T. Biosynthesis of parathyroid hormone, N. Eng. J. Med 1978; 299, 635. |
| [22] | John W. Rowe, and Barbara A.Gilchrest. Cutaneous aspects of renal disease. ed. 2 New York Mc Graw - Hill Bookco pp 1979; 1408 –1411. |
| [23] | Barbara A. Gilchrest, John W., Rowe and Martin C.M. Clinical and histological skin changes in chronic renal failure. Lancet 1980; 2.1271-1275. |
| [24] | Cawly E.P. The eccrine sweat glands of patients in uremia. Arch. Dermatol 1961; 84:51 –58. |
| [25] | Bartman I. and Landing. 13.14: (1966). Morphology of sweat apparatus in cystic fibrosis. Amer. J . Clin. Path. 54: 455 - 459. |
| [26] | Stanburry S.W., (1971). Calcium and phosphorus metabolism in renal failure. Diseases of the Kidney. 2nd.ed. 305 -333. |
| [27] | Arnaud C.D. (1973). Hyperparathyroidism and renal failure . Kidney. Int. 4: 89. |
| [28] | Vosik W. M. Successful medical mangement of osteitis fibrosa due to tertiary hyperparathyroidism. Mayoclin. Proc 1972; 47, 110. |
| [29] | Massry S.G., Broutbar N., and Goldstein D.A. Ca + P in health and CRF. Nephrology an approach to the patient with renal disease. J.B., Lippincott company 1982; P.154 –179. |
| [30] | Muligan R. M. Metastatic calcification. Arch. Path 1947. 43: 177–230. |
| [31] | Putkonen T and Wangel G. A. Renal hyperparathyroidism with metastatic calcification of the skin. Dermatologica,118: 127-144. |
| [32] | Parfitt A. M. (1969). Soft tissue calcification in uremia. Arch . intern. Med 1959. 124: 544 –556. |
| [33] | Eisenberg E. and Bartholow P. V. Reversible calcinosis cutis: calciphylaxis in man. New. Eng. J. Med 1963; 268: 1216-1220. |
| [34] | Massry S.G. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia: Disappearance of itching often subtotal parathyroidectomy. New. Eng. J. Med 1968; 279: 697 –700. |
| [35] | Gayler B.W. and Brogdon B.G. Soft – tissue calcification in the extremities in systemic disease. Amer. J. Med. Sci 1965; 249: 590 –605. |
| [36] | Longan M.A. Advances in the chemistry of calcification. Physiol. Rev 1940; 20: 522 – 560. |
| [37] | Walser M. The chronic composition of uremic plasma. Abstracted, J. Clin. Investigation 1959; 38: 1052. |
| [38] | Herbert F. K., Miller H. G. and Richardvon G. O. Chronic renal disease, secondary parathyroid hyperplosia, Decalcification of bone and metabolic calcification. J. Path. Bact 1941; 53: 161–182. |
| [39] | Ress J. K. and Coles G. A. Calciphyloxis in man. British Med. J. 1969; 2: 670– 672. |
| [40] | Selye H. Tuchweber B and Gabbiani G. Acta. Endocrinologica, suppl 1964; 90: 203. |
| [41] | Anderson D.G. Stewart W. K. and Pievcy D. M. Lancet 1968; 2: 323. |
| [42] | Copp D. H. Calcium and phosphorus metabolism. Amer. J. Med 1957; 22: 275–285. |
| [43] | Urist M. R., Moss M. J., and Adams J. M. Calcification of terdon: A triphosic local mechanism. Arch. Path 1964; 77: 594 – 608. |
| [44] | David G. Adesonm William K. Stewart, and David M. Pievry. Calcifying panniculitis with fat and skin necrosis in a case of uremia with hyperparathyroids. Lancet 10 August 1968; 323 –325. |
| [45] | Friedman M., Marshall – Jones P. and Ross E. J. Q. J. Med 1966; 35: 193. |
| [46] | Olmostead E. G. , and Lunseth J. H. Skin manifestations of chronic acidosis. Arch. Dermatol 1958; 77: 304 – 314. |
| [47] | Worth R.L. Calciphylaxis: Pathogenesis and therapy. Cutan Med Sung Apr 1998; 2 (4): 245 – 8. |
| [48] | Roe – SM, Graham – L.D. Brock – W.B., and Barker – D.E. Calciphylaxis: Early recognition and mangement. Am – Sung 1994 Feb; 60 (2): 81 – 6. |
| [49] | Hohl D. Necrotizing vasculan calcinosis. MEDLINE Dermatology 1994; 189: 432 – 4. |
| [50] | Benkalfate L., Zein K., Le – Gall F., Chevvant – Breton J., Rivalan J, and Le – Pogamp P. Calcified intertrigo; a vare cause of cutaneous calcinosis. Ann–Dermatology–Venereol 1995; 122: 789 – 92. |
| [51] | Tan H .H., and Cheong W. K. Cutaneos gangvene secondary to metastatic calcification in end stage renal failure. Singapore – Med – J 1996 Aug.; 37: 438 – 40. |
| [52] | Uchida M., Sakemi T., Ikeda Y., and Maeda T. Acute progressive and extensive metastatic calcification in a nephrotic patient following chronic hemodialysis. Am. J. Nephrol 1995; 15: 427–30. |
| [53] | Gilkes J.H., Eady R. A., and Lesley H. Ress. Plasma immunoreactive melanotrophic hormones in patients on mainterarce haemodialysis. British Medical Journal 1 1975; 656 – 658. |
| [54] | Smith A. G., Sam Shouster and A. J.Thody. Role of the kidney in regulation plasma immunoreactive beta – melanocyte stimulating hormone. British medical journal 1 1976; 874 – 876. |
| [55] | Abe K. Levels of immunoreactive B. MSH in CRF. British J. of clinical investigation 1969; 48: 1580. |
| [56] | Shuster S., and Thody A. J. Immunoreactive B. MSH. Journal of investigation Dermatology 1974; 62: 172. |
| [57] | Hmida M. B., Turki H., Hachicha J., Reygagne P., Rabier D., Zahaf A., and Jarraya A. Hypopigmentation in hemodialysis. MEDLINE Dermatology 1996; 192:148 – 52. |
| [58] | Kalman Keczkes and Malcolm Farr. Bullouss dermatosis of chronic renal failure. British journal of Dermatoloy 1976; 95: 541–546. |
| [59] | Maureen Poh -Fitzpatrick. Bullous disease of dialysis: Topic Last Updated 11/3/ 2000; 11: 54: 20. |
| [60] | Mantoux F., Bahadovan P., Perrin C., Bermon C., Lacour J.P., and Ortonne J. P. Flutamide –induced late cutaneous pseudoporphyra. Ann–Dermatology–Venereol 1999 Feb.; 126:150 – 2. |
| [61] | Glynne P., Deacon A., Goldsmith D.; Pusey C., and Clutterbuck E. Bullous dermatosis in E .S .R .O: Porphyria on pseudoporphyria . Am. J. kidney. Dis 1999 Jul.; 34:155 – 60. |
| [62] | Kenedy A. C. , and Lyell A. Accquived epydermolysis bullosa due to high dose of frusemide. British medical journal 1 1976; 1509 -1510. |
| [63] | Kalman Keczkes and Malcolm Farr. Cutaneous bullous and frusemide in CRF. British medical journal 1972; 2: 236. |
| [64] | Shaul G. Massry, Mordecai M., Poportzen and Jake W. Coburn. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. New England Journal of Medicine 1968; 279: 697 –703. |
| [65] | Berson S. A. and Yallow R. S. Parathyroid hormonein plasma in adenomatous hyperparathyroidism. Science 1966; 154 : 907. |
| [66] | Dimkovic N., Djukanovic L., Radmilovic A., Bojic P., and Juloski T. Uremic pruritus and skin most cells. Nephron 1992; 61: 5 – 9. |
| [67] | David Maclachlan and Alexander L. Forrest: (1974). Itching in renal failure. Lancet, 2: 355. |
| [68] | Barbara A. Gilchrest, John W. Rowe and Robert S.Brown. Relief of uremic pruritus with ultraviolet phototherapy.Lancet 1977; 297:136–138. |
| [69] | Eugene L. Saltzer. Relief from uremic pruritus: A therapeutic approach. Cutis 1975; 16: 298 –299. |
| [70] | Yatzidis H., Digenis P., and Tountas C. Heparin treatment of uremic itching. Jama 1972; 222:1183. |
| [71] | Silverberg D. S., Iaina A., and Reisin E. Cholestyramine in uremic pruritus. British medical journal 1 1977; 752 – 753. |
| [72] | Luis Tapia, Jhoong S. Cheigh, and David S. David. Pruritus in dialysis patients treated with parentral lidocaine. New Enngland Journal 1977; 296: 261–262. |
| [73] | Constantine L. Hampers, Adrian I. Katz and Richard E.Wilson. Disappearance of uremic itching after subtotal parathyroidectomy. New England Journal 1968; 279: 695–697. |
| [74] | Robertson K.E. et al. Uraemic pruritus, Am. J. Health . Syst. Pharm 1996. 53: 2159. |
| [75] | Muchrcke R. C. The finger nails in chronic hypoalbuminemia. British Medical Journal 1 1956; 1327–1328. |
| [76] | Samuel L. Moschella, Donald M. Pillsbury and Harvy J. Hurty. Diseases of nutrition and metabolism. Dermatology vol II. W. B. Saunders company. Philadelphia, London, Toronto P 1975; 1237 – 1322. |
| [77] | James B. Hudson and Allen J. Dennis. Transverse white lines in the finger nails after. Acute and chronic renal failure. Arch. Intern. Med. Vol 1966.117: 276–279. |
| [78] | Philip G. Lindsay. The half and half nail. Arch. Intern. Med 1967; 119: 583–587. |
| [79] | James J. Leyden and Margared Gray Wood.“ The half–and –half nail ``A uremic onychopathy. Arch. Derm. Vol 1972; 105: 591–592. |
| [80] | Lindsay P. G. The half and half nail. J. Lab. Clin. Med. 1965; 66: 82. |
| [81] | Lubach D., Strubbe J. and Schmitt J. The half and half nail phenomenon in chronic hemodialysis patients. Dermatologica 1982; 164: 350–353. |
| [82] | Constantine V. S., and Carter V. H. Kyrle’s disease: Histopathologica Findings in five cases and review of the literatune. Arch. Dermatol 1968; 97: 633–639. |
| [83] | Antoinette F., Gray L. Hardegen and Alfredo R. Zarate. Kyrle’s disease in patients with CRF. Arch. Dermatol 1982; 118: 85–88. |
| [84] | Patterson J.W. The perforating disorders. J. Am. Acad. Dermatol 1984; 10: 561. |
| [85] | Mehta R. L. Skin necrosis associated with acquired protein C deficiency in patients with renal failure and calciphylaxis. Am J. Med 1990; 88. |
| [86] | Goldblum O. M. Pseudo – kaposi sarcoma of the hand associated with an acquired, iatrogenic arteriovenous fistula. Arch. Dermatol 1985; 121: 1038. |
| [87] | Fuchs E. Dialysis acne. J. Am. Acad. Dermatology 1990; 23:125. |
| [88] | Rudlinger R. Human papilloma virus infections in a group of renal transplant recipients. Br. J. Dermatology 1986; 115: 681. |
| [89] | Gupta A.K. Cutaneous malignant neoplasms in patients with renal transplants. Arch. Dermatology 1986; 122: 1288. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML