-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2016; 5(1): 6-15
doi:10.5923/j.ajdv.20160501.03

Efficacy and Safety of Two Doses of Sphaeranthus indicus Extract in the Management of Plaque Psoriasis: A Randomized, Double Blind, Placebo Controlled Phase II Trial
Sangeeta Velaskar 1, Chitra S. Nayak 1, Raghunandan G. Torsekar 2, Vishalakshi Viswanath 2, Uday Khopkar 3, Vinay Saraf 4, Sujay Kulkarni 5, Amol Shindikar 5, Ajit Patil 5, Sajjad Patil 5, Himanshu Parikh 5, Ashish C. Suthar 6, Vijaysingh Chauhan 6, Muralidhara Padigaru 7, Debashish Chakrabarti 7, Somesh Sharma 8
1Dept of Dermatology, BYL Nair Hospital and TN Medical College, Mumbai, India
2Dept of Dermatology, Chhatrapati Shivaji Maharaj Hospital and Rajiv Gandhi Medical College, Thane, India
3Dept of Dermatology KEM Hospital & Seth GS Medical College, Mumbai, India
4Professor and Senior Consultant Dermatologist, Skin Clinic, Goregaon, Mumbai, India
5Clinical Research and Development, Piramal Enterprises Limited, Goregaon-East, Mumbai, India
6Phytomedicines Department, Piramal Enterprises Limited, Goregaon-East, Mumbai, India
7Biomarker Department, Piramal Enterprises Limited, Goregaon-East, Mumbai, India
8CEO, Piramal Enterprises Limited, Goregaon-East, Mumbai, India
Correspondence to: Ashish C. Suthar , Phytomedicines Department, Piramal Enterprises Limited, Goregaon-East, Mumbai, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Sphaeranthus indicus (S. indicus) is traditionally used in treatment of various diseases; in vitro study showed that it inhibited release of inflammatory cytokines. Objectives: To evaluate the efficacy and safety of 12 weeks course of oral tablet of S. indicus extract (Tinefcon® ) in plaque psoriasis patients. Methods: A total of 74 patients with moderate to severe plaque psoriasis were randomized in (1:1:1) ratio to three arms placebo, S. indicus extract 1.4 g/day (low dose) and 2.8 g/day (high dose). Treatment was given for 12 weeks. Patients were evaluated for Psoriasis Area Severity Index score (PASI), Physician’s Global Assessment (PGA) along with histopathological analysis, gene expression profile and safety. Results: At week 12, 65% patients achieved PASI-50 and 40% patients achieved PASI-75 in high dose group also in same group PASI 90 was achieved by 15% patients. PASI-50 and PASI-75 response was achieved in 31% and 23% of patients respectively, in low dose group. In placebo group PASI-50 was reached by 57% patients and PASI-75 by 14% patients. Improvement in PGA status was observed in 80% patients and 62% patients treated with S. indicus extract high and low dose group respectively. Histopathological and biomarker data strongly support the improvement in the disease severity. Conclusions: S. indicus extract (Tinefcon® ) was safe and well tolerated in both the dosage strengths and was more effective in subjects with moderate to severe plaque type psoriasis at the higher dosage of 2.8 g/day.
Keywords: Psoriasis, Sphaeranthus indicus, Psoriasis vulgaris
Cite this paper: Sangeeta Velaskar , Chitra S. Nayak , Raghunandan G. Torsekar , Vishalakshi Viswanath , Uday Khopkar , Vinay Saraf , Sujay Kulkarni , Amol Shindikar , Ajit Patil , Sajjad Patil , Himanshu Parikh , Ashish C. Suthar , Vijaysingh Chauhan , Muralidhara Padigaru , Debashish Chakrabarti , Somesh Sharma , Efficacy and Safety of Two Doses of Sphaeranthus indicus Extract in the Management of Plaque Psoriasis: A Randomized, Double Blind, Placebo Controlled Phase II Trial, American Journal of Dermatology and Venereology, Vol. 5 No. 1, 2016, pp. 6-15. doi: 10.5923/j.ajdv.20160501.03.
Article Outline
1. Introduction
- Psoriasis is a potentially life-threatening, genetic, immune-mediated chronic inflammatory disease expressing in the skin or joints or both [1-4]. As per World Psoriasis Day consortium 2014, psoriasis affects more than 125 million people worldwide [5]. The prevalence of psoriasis was found 2- 4.6% in US, 4.7% in Canada, 1% in Asia, and 1.87% in UK [6-9]. In India prevalence of psoriasis was 0.4-2.8% with male to female ratio 2:1 [7]. Psoriasis adversely affects all aspects of life like social, physical, emotional, work capacity and financial aspects with a serious negative impact on quality of life; risk of suicidal ideation with psoriasis patients is also high [8-12]. Patients with psoriasis usually become victims of depression, anxiety, diabetes and cardiovascular disorders [13]. Psoriasis treatment incurs serious financial burden, annual cost of psoriasis was more than € 7 billion in Germany and $ 112 billion in the United States [14, 15].Generally, there are 5 types of psoriasis namely plaque, guttate, inverse, pustular and erythrodermic psoriasis. Most commonly observed psoriasis is plaque psoriasis which is also known as psoriasis vulgaris, it affects 80 to 90% of overall patients with psoriasis. Raised, red, thick patches on the skin with silvery white scales are characteristics of plaque psoriasis [16, 17]. Both the innate immune system and adaptive immune system are involved in immunopathogenesis of psoriasis [18, 19]. Cytokine tumor necrosis factor-α (TNF-α) plays a very crucial role in pathogenesis of psoriasis. TNF-α is involved in keratinocyte proliferation, increased secretion of chemoattractant and metabolic dysregulation [20, 21]. Increased level of TNF-α is seen in psoriatic lesion as compared to natural skin of psoriatic patients [22]. TNF-α antagonist are known to be effective treatment modality for psoriasis [20]. Albeit management of psoriasis has a plethora of options, however they have disadvantages [23]. Topical therapy contains emollients, corticosteroid, vitamin D analogues, calcineurien inhibitors like tacrolimus and pimecrolimus, retinoids and tars [24]. Topical preparations work well only in mild to moderate psoriasis. It faces problem of inconvenience and serious side effects. Long term use of corticosteroid cause adverse effects like epidermal atrophy, acneiform eruptions and in rare case causes suppression of the hypothalamic pituitary-adrenal axis [24]. Conventional systemic therapy has limited use because of associated toxicity; cyclosporine hampers renal function, methotrexate causes ulcerative stomatitis, myelosuppression and hepatotoxicity. Acitretin is classified as pregnancy category X, in high dose it leads to liver damage [8, 23, 24]. Prolonged use of Psoralen Ultraviolet A (PUVA) is associated with significant photo-aging and an augmented risk of skin cancer [24]. Biologics emerged as promising option but they are expensive and risk of lymphoma, increased chances of opportunistic infection are allied with their use [25, 26]. Developments of anti-drug-antibodies (ADAs), serious and fatal adverse events are the limitation with the use of biologics [27].Now a day’s herbal treatment is favored for psoriasis management because of excellent efficacy profile, less side effects, ready availability, low cost and ease of use [28, 29]. Chances of drug-herb interactions are less [30] so herbal treatment can be used along with other psoriasis modalities. Sphaeranthus indicus Linn (Family: Astracea) is medicinal plant used traditionally to treat several diseases [31]. S. indicus possesses anti-inflammatory and immunomodulatory activity, extract of which shows inhibition of the release of TNF-α and IL-12/23 [31, 32]. In in vitro and in vivo experiments conducted by Piramal Life Sciences Limited, S. indicus extract has inhibited release of TNF-α, IL–1β, IL-6 and IL-8 [32]. These studies provide novel molecular insights into the anti-inflammatory, anti-migratory and anti-proliferative properties of S. indicus and show that it can be used as a therapeutic option in inflammatory and auto-immune conditions such as psoriasis. The present study was conducted to evaluate the safety and efficacy of oral tablets of two doses of S. indicus extract in patients with moderate to severe psoriasis.
2. Materials and Methods
2.1. Patients
- Patients having plaque psoriasis more than 6 months or at least 6 months prior to screening were recruited from 4 centers of India. Patients with 18-70 years of age, having PASI score >10 even after at least one month treatment with topical steroids or for 2 months with other topical agents used in the treatment of psoriasis were included in the study. Women of childbearing potential and men were instructed to use adequate contraception prior to entry into study, during the study and 4 weeks after withdrawal from the study. Subject were excluded if they had received systemic therapy such as Methotrexate or Cyclosporine in the past three months prior to randomization or received TNF-α inhibitor therapy any time in the past prior to screening, patients with spontaneously improving or rapidly deteriorating plaque psoriasis, acute guttate psoriasis, pustular, inverse or erythrodermic psoriasis, psoriatic arthritis, subject with serious dermatological infection in past three months requiring systemic therapy.The study protocol and amendments were approved by the Institutional ethics committees and was registered in ctri.nic.in (CTRI Number: CTRI/2007/091/000003). Written informed consent was obtained from all the patients.
2.2. Plant Material and Drug Preparations
- The extract was prepared from fresh flowering and fruiting heads of S. indicus which were collected from Kelwe Road, Maharashtra, India and were authenticated in Piramal Life Sciences Limited, Mumbai, India. A voucher specimen (No. Herb-00230) was placed in the herbarium of Piramal Life Sciences Limited. The extraction and preparation of extract was performed as described in a prior publication [33]. Each tablet contained 700 mg of S. indicus extract; the percentage of quantified chemical constituents per tablet was as follows: not less than (NLT) 28 mg of 7-hydroxy frullanolide and 9.6 mg of Sphaeranthanolide. The high-pressure liquid chromatography chemical fingerprint for the extract of S. indicus can be seen in the Figure 1.
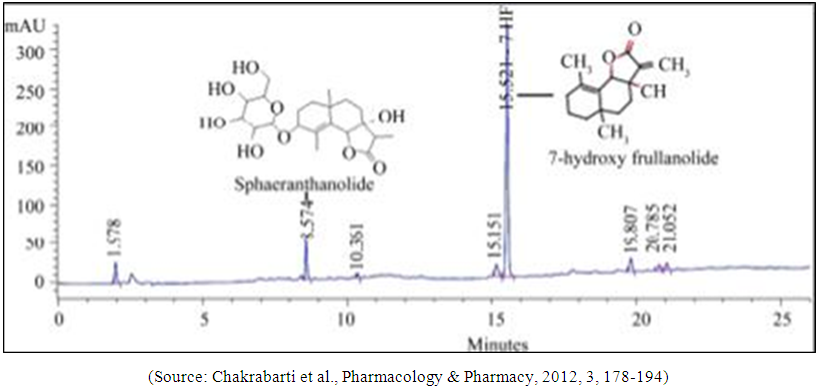 | Figure 1. HPLC fingerprint |
2.3. Study Design
- This study was a randomized, double blind, placebo controlled, pilot study to evaluate the safety and efficacy of 2 doses of S. indicus extract (Tinefcon® ) in patients with moderate to severe psoriasis. Total 74 patients were randomized in 1:1:1 ratio in placebo, high dose of S. indicus extract and low dose of S. indicus extract. Twenty four patients received placebo and 25 patients each, received 2.8 g/day (2 tablets of 700 mg twice daily) and 1.4 g/day (1 tablet of 700 mg twice daily) of S. indicus extract for 3 months. It was advised to be taken after 30 minutes of breakfast and dinner. Patients were prohibited to use investigational or commercial agents or therapies for psoriasis but use of emollients and chlorpheniramine maleate were allowed. Drugs which provoke psoriasis like beta blockers, ACE Inhibitors, non-steroidal anti-inflammatory drug, anti-malarial and antihistaminics (except chlorpheniramine maleate) were contraindicated during study period. At the time of screening, physical examination, vital parameters, PASI score were assessed within 10 days of signing Informed consent along with other haematological and biochemical parameters. One month wash out period was given for patients receiving PUVA therapy in less than 4 weeks prior to consenting or 2 weeks if the subject had been receiving topical therapy in less than 2 weeks prior to consenting.
2.4. Efficacy Measures
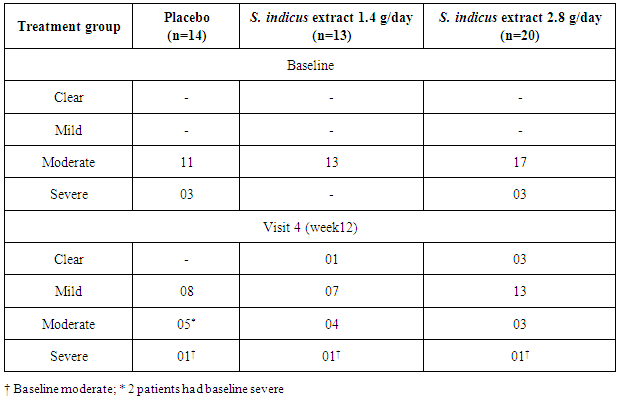
- The primary efficacy variables were determination of safety of S. indicus extract and effects on PASI score in patients with moderate to severe psoriasis. Secondary efficacy variables include assessment of changes in histopathology of psoriatic skin and gene expression profile study using DNA micro array estimation from the skin biopsy specimens. The PASI score was calculated according to the standard method outlined by Fredriksson and Petersson at baseline [34]. Change in PASI score at visit 4 (week 12) compared to the baseline was calculated. Proportion of patients with an improvement in the index of at least 50% and 75% were determined. For assessment of Physician’s Global Assessment (PGA), physicians evaluated psoriatic skin lesions for disease activity as mild, moderate, severe or clear.
2.5. Histopathology Evaluation
- Histopathological assessment of the psoriatic skin lesion was performed at baseline and visit 4 (week 12). Epidermal thickness, or hyperkeratosis, parakeratosis, hypogranulosis and inflammatory cells evaluated in epidermis while in dermis, papillary capillary dilation and inflammatory cells was evaluated. Hematoxylin and eosin stain (Vector Laboratories, Burlingame, CA) was used to stain skin biopsy samples. Skin biopsy samples were obtained from same target lesion (from different parts at subsequent visits) at the baseline and at the end of the treatment visit 4 (week 12). The biopsy sample was collected from thickest (central) part of target lesion excluding bony surface and flexure. Histopathologist was blinded to the slides; evaluation was done at a central laboratory. Epidermal thickness was measured using Image Pro software and expressed as micrometer (μM) thickness.
2.6. Immunohistochemistry
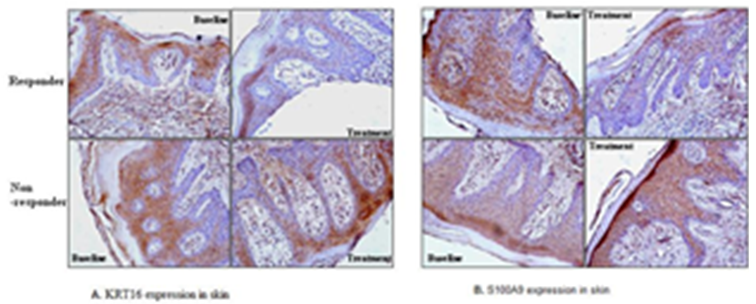
- Immunohistochemical profiling was also done to study protein expression for psoriatic markers such as keratin 16 (KRT16), S100A9 and measurement of the infiltrating inflammatory cells [dendritic cells (CD11c)] was done for determination of molecular response. For immunohistochemical assessment the paraffin embedded sections were deparaffinized, rehydrated and antigen retrieval was carried out. Primary antibodies against keratin 10 and S100A9 (Santa Cruz Biotechnologies, Santa Cruz, CA) were applied on the slide followed by immunofluorescence method using DyLite 549 conjugated secondary antibody. Hoechst 33342 (Thermo Fisher Scientific, Waltham, USA) was used for nuclear staining. Epidermal thickness was measured and quantified using Nikon 80i microscope and Image Pro Plus software (Media Cybernetics, Silver Springs, CA).
2.7. Gene Expression Profile
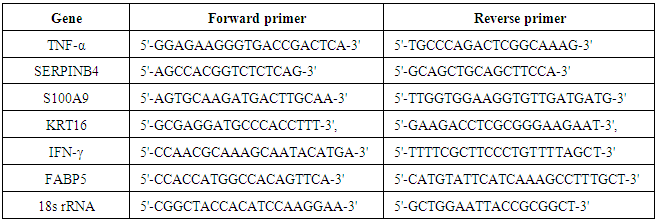
- Expression of genes like peptidase inhibitor 3 (PI3), KRT16, TNF-α, fatty acid binding protein 5 (FABP5), S100A9, serine protease inhibitor B4 (SERPINB4) and interferon–gamma (IFN-γ) were studied from skin biopsy samples by quantitative polymerase chain reaction (qPCR) at biomarker discovery department of Piramal Life Sciences Limited, Mumbai. Numbers of patients in non-responder and responder category were calculated. Patients’ group was defined as ‘Responder’ when there was a down regulation of gene expression for more than 5 genes and ‘Non-Responder’ when there were no changes in the expression levels for more than 5 genes.
2.8. Randomization
- This was a double-blind randomized study. The patients were randomized by permuted block randomization. Centralized concealment blinded to participant, investigator, outcome assessor and data entry operator.
2.9. Statistical Tests
- Pearson Chi–Square test was used to determine significance amongst proportion of patients with improvement in PASI score. The Analysis of variance (ANOVA) test was performed on change in PASI score from baseline to visit 4 with group (treatment) as factor. Repeated measure ANOVA was performed to determine any evidence of a difference in the response profiles between the placebo and the two dose levels. Immunohistochemistry data was analyzed using Student’s t test and expressed as mean ± SD. Statistical significance was assumed for p<0.05.
3. Results
3.1. Demographics
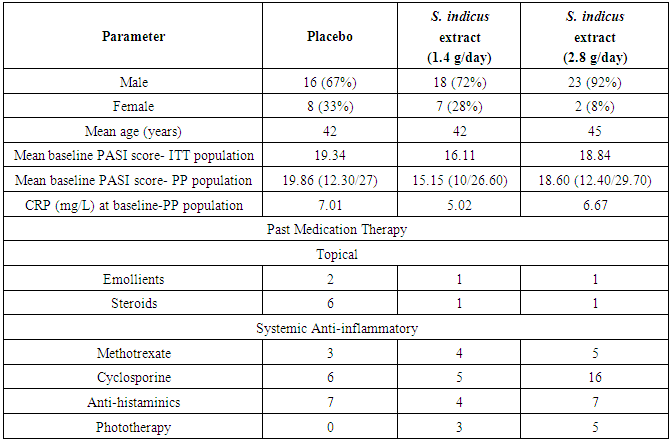
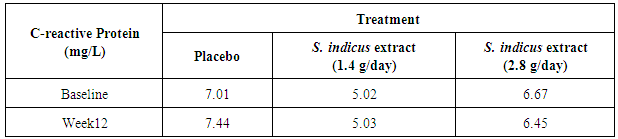
- Total 74 patients were randomized in this study. From 74 patients, 17 patients were females and 57 patients were males (Table 1 and Figure 2). Forty eight out of 74 patients completed the study, 14 patients from placebo, 20 patients from 2.8 g/day S. indicus extract and 14 from 1.4 g/day S. indicus extract (Figure 2). Number of patients discontinued the treatment was more in placebo group than in 2.8 g/day S. indicus extract group. There was no major difference between three groups in terms of age, mean baseline PASI score and mean baseline C reactive protein CRP (mg/ml) level. Most commonly used prior treatment was topical steroids and emollients, systemic methotrexate and anti-histaminics, including phototherapy.
|
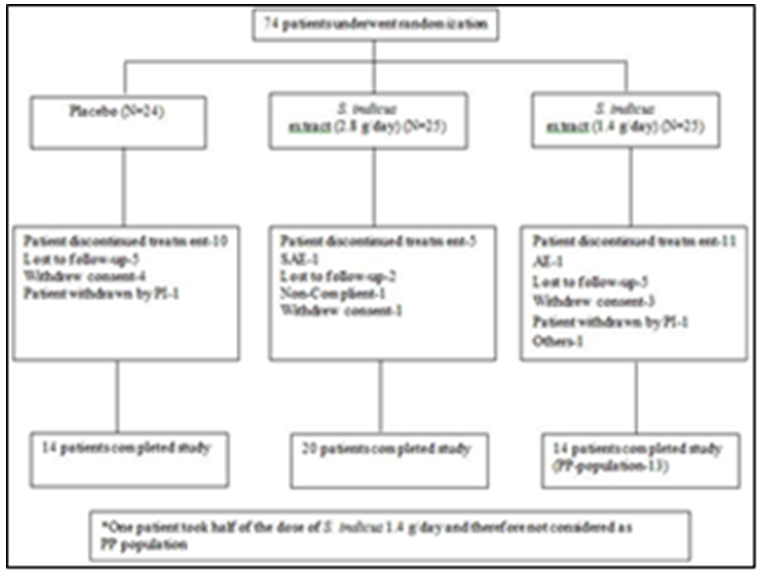 | Figure 2. Subject disposition |
3.2. Efficacy Measures
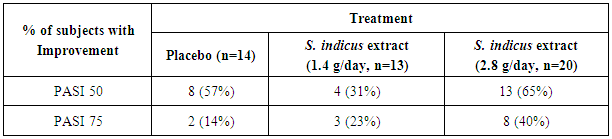
- Psoriasis Area Severity Index ResponseAfter 12 weeks of treatment, three (23%) of 13 patients treated with low dose S. indicus extract and 8 (40%) of 20 patients treated with high dose S. indicus extract achieved PASI 75 response; in placebo group 2 (14%) patients achieved PASI 75 out of 14 patients (Table 2). PASI 50 response was achieved by 13 (65%) of 20 patients, 4 (31%) of 13 patients from S. indicus extract high dose treated group and S. indicus extract low dose treated group respectively as compared to 8 (57%) of 14 patients of placebo group.
|
|
3.3. Histopathology Analysis
- Histopathology analysis showed marked improvement in 5 patients in low dose group and 4 patients in placebo group while 7 patients indicated marked improvement in high dose group (Figure 3). In patients showing improvement, complete absence of parakeratosis and neutrophils; restoration of granular layer; reduction in rete ridge elongation in epidermis, reduction in dilation of papillary capillaries and absence of neutrophils in dermal infiltrate was noted. In general, improvement was observed in 15 patients in high dose group, 7 patients in low dose group and 9 patients in placebo group. No patients in high dose group of S. indicus extract showed moderate worsening whereas 1 patient from low dose group and 2 patients from placebo group showed moderate worsening. Mild worsening was seen in all the groups.
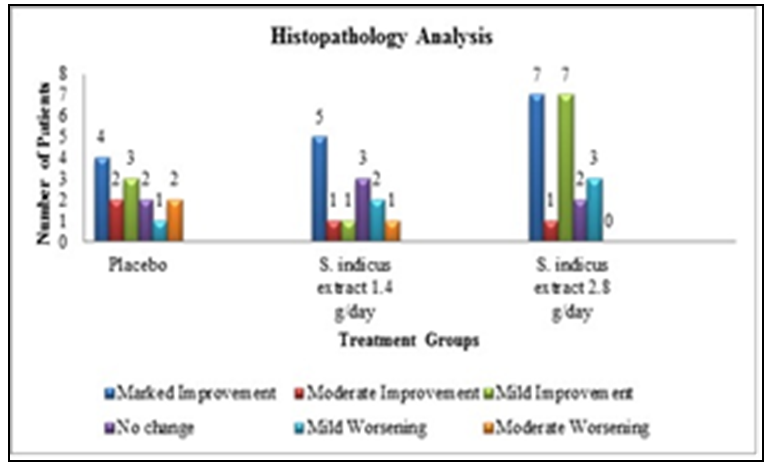 | Figure 3. Histopathology analysis |
|
3.4. Gene Expression Profile
- Gene expression profiling (TNF-α, SERPINB4, S100A9, SKALP, KRT16, IFN-γ and FABP-5) revealed response in 23 patients out of which 14 (61%) patients in 2.8 g/day group, 5 (22%) in 1.4 g/day group and 4 (17%) patients treated with placebo. Twenty two patients were non-responders (Figure 4 and 5). PCR Primer sequences used in the gene expression study are given in Table 5.
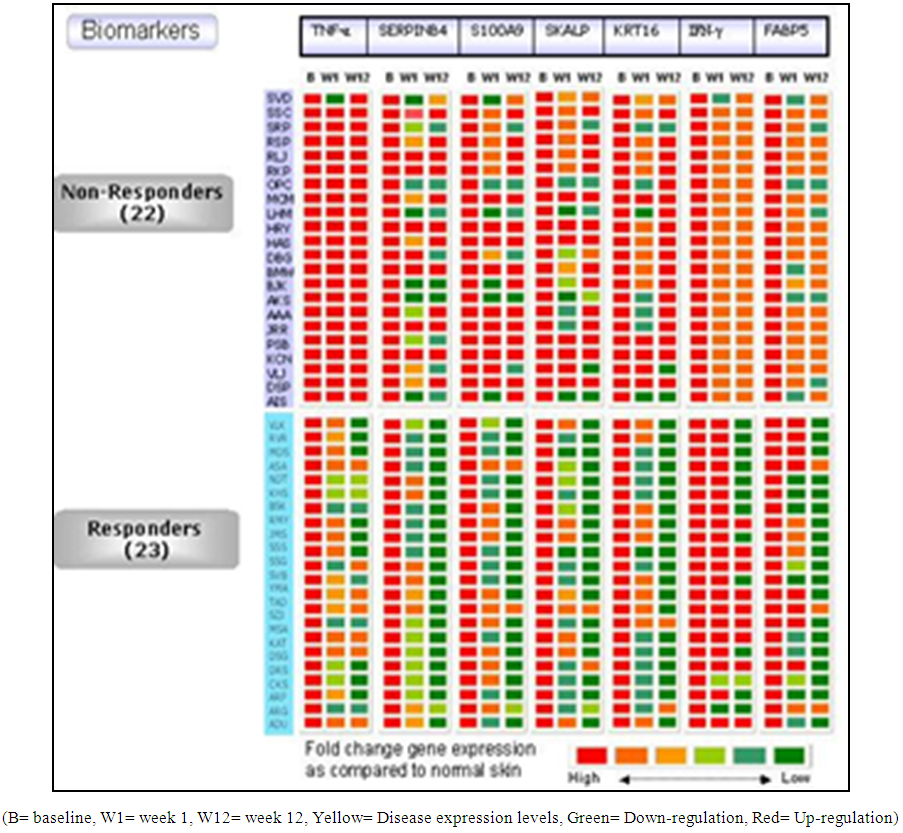 | Figure 4. Heat map for gene expression profile as response to S. indicus extract |
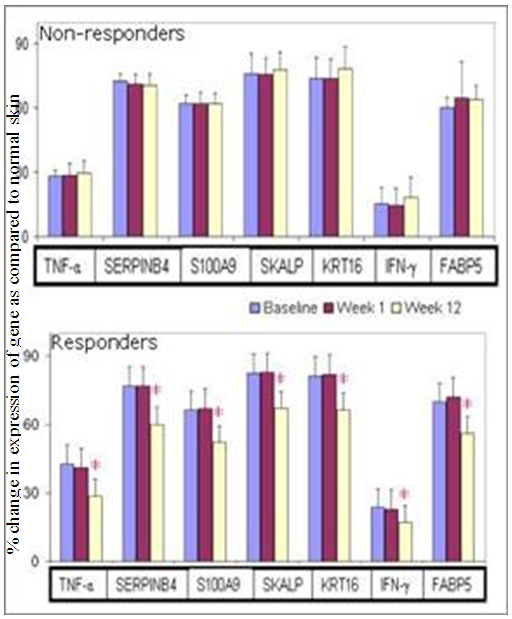 | Figure 5. Gene expression profile for psoriatic markers in Responders and Non-Responders |
|
3.5. Immunohistochemistry
- Epidermal ThicknessAfter 12 weeks of treatment, epidermal thickness was significantly decreased as compared to baseline in 12 (75%) patients (responders) out of 16 patients (p<0.001), whereas 4 (25%) patients did not show reduction (non-responders). Out of the 12 responder patients, 50% patient received 2.8 g/day S. indicus extract, 33% patients received 1.4g/day of S. indicus extract and 17% patients received placebo. All four non-responders received placebo. Patient 1 and 2 represent responder; and patient 3 was a non-responder (Figure 6). The 16 patients were randomly selected from the studied population for epidermal thickness characteristic. These results indicate that the efficacy of the product increases with the dose.
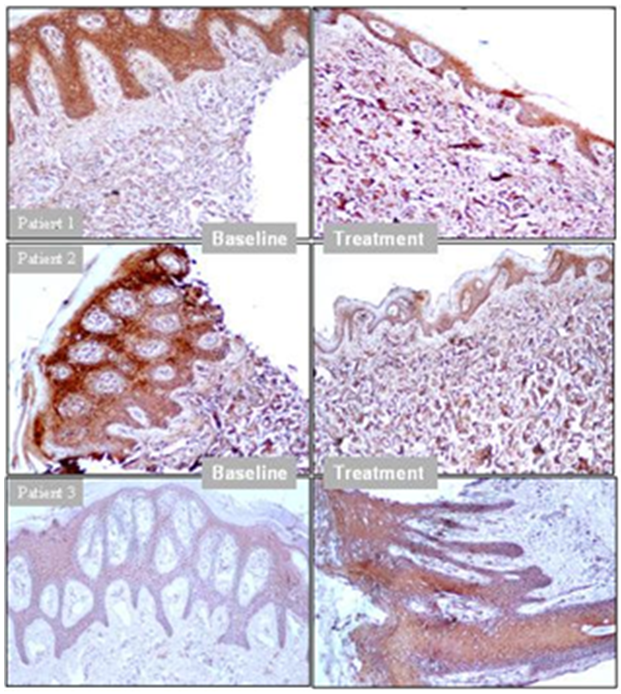 | Figure 6. Epidermal thicknesses as a measure of active disease and drug response to S. indicus extract (Patients 1, 2 = Responders, Patient 3 = Non-responder) |
3.6. Safety Analysis
- Except for three serious adverse events (SAE) (exacerbation of psoriasis, erythroderma and urinary tract infection) that occurred in this study, all other adverse events were not-serious. There was no mortality noted. One event of hyperglycemia and 1 event of hyperchlorhydria were observed in S. indicus extract low dose and high dose group respectively. Out of three SAE, two SAE were from high dose group of S. indicus extract viz exacerbation of psoriasis (increase in erythema, scaling and induration of existing lesion) in a 41-year-old female and urinary tract infection (UTI) in a 61-year-old male subject at exit visit. S. indicus extract, of these only UTI was possibly related to S. indicus extract as per Investigator’s assessment. When ultrasonography carried out in the patient with UTI, it revealed pyonephrosis and benign prostatic enlargement (38 cc of prostatic volume). The patient was hospitalized and around 500 ml of pus was drained and antibiotics were administered. Erythroderma was developed in 49 year old male receiving 1.4 g/day of S. indicus extract. The subject complained of increase in erythema, scaling and induration of existing lesion with development of new lesions. There was a history of moderate fever. On examination, subject was found to have generalized erythema and scaling. The subject was hospitalized for further management of erythroderma. As per Investigator’s assessment the SAE was unlikely related to S. indicus extract.
4. Discussion
- Outcomes of the present study revealed that S. indicus extract (Tinefcon® ) was effective in reducing the severity of psoriasis. Dose dependent improvement in all the parameters was observed in S. indicus extract treated group. PASI 75 response was achieved by 23%, 40% and 14% in S. indicus extract 1.4 g/day, S. indicus extract 2.8 g/day and placebo groups, respectively. PASI 50 was reached by 31% and 65% patients in S. indicus extract low dose and high dose group respectively. Improvement in psoriasis was observed not only by PASI, PGA score but also with histopathological, immunohistochemistry and gene expression analysis. Assessments of PGA were consistent with PASI results; patients receiving S. indicus extract achieved ‘‘clear’’ or ‘‘mild” status after 12 weeks. Out of 20 patients from the S. indicus extract high dose group, 80% patients achieved clear or mild status while out of 13 patients from the S. indicus extract low dose group, 62% patients achieved clear or mild status.Histological features of plaque psoriasis include hyperkeratosis mainly composed of parakeratosis some orthrokeratosis; neutrophils in the epidermis; regular acanthosis often with clubbed rete ridges, dilated capillaries in dermal papillae [35, 36]. From patients showing improvement, histopathological analysis showed improvement in parakeratosis and epidermal thickness, neutrophils were totally absent, granular layer was found to be restored; elongation of rete ridge in epidermis was decreased also reduction in dilation of papillary capillaries and absence of neutrophils in dermal infiltrate was detected. Presence of CD11c positive cell are more in psoriatic skin than the normal skin, it also plays pivotal role in initiation of the plaque in the perilesional skin along with other dendritic cells subsets [37]. Reduction in epidermal thickness with decrease in infiltration of CD11c-positive cells and down regulation of inflammatory marker genes (KRT16, TNF-α, FABP5, S100A9, SERPINB4 and IFN-γ) helps to understand the cellular effect and molecular level effect of S. indicus extract in psoriasis. Biomarker studied in this study provides better prediction about severity of psoriasis. Further studies on correlation between these markers and PASI score for determining disease severity and treatment response will be highly interesting and will be useful for better management of psoriasis. Improvement in all these parameters occurred within short duration of treatment. Some of the patients were non-responders to the treatment and this observed feature was not specific to any gender, race or other obvious reasons. As the treatment was plant based and studied for a period of 3 months, this period may be in-sufficient for these non-responders to show the response and they may have responded if the treatment was extended and studied further.The role of TNF-α, IL-6, IL-8 is very well known in pathogenesis of psoriasis [38]. In previous in-vitro study with S. indicus extract showed inhibition of cytokines such as TNF-α, IL-6, IL-1β, IL-8, [33] thus inhibition of pro-inflammatory cytokines is one of the mechanism of anti-psoriatic activity of S. indicus extract. The previously studied in vitro experiments show that Tinefcon has an inhibitory effect on TNF-α and regulates the NFκB pathway triggered by TNF-stimulation. Tinefcon lowers the nuclear translocation of p65 sub-unit in macrophages and thus reduces NFκB mediated transcription process.Similar results observed with other TNF-α antagonist like etanercept. Gottelib et al. showed that 24 weeks of treatment with subcutaneous Etanercept 25 mg twice weekly; 60% patients achieved PASI 50 after 3 months and 60% patients achieved PASI 75 after 6 months, thinning of the epidermis and absence of KRT16 in suprabasal keratinocytes, reduction in CD11c-positive cells was also observed in this study [39].The safety data observed in this study revealed that S. indicus extract had good tolerability profile. Most of the adverse events were mild to moderate and no death was observed. Out of 3 SAE only one SAE was possibly related to S. indicus extract which was pyonephrosis that occurred in S. indicus extract high dose group. All the patients in S. indicus extract had normal renal and liver function.Limitation of the study was a small sample size. Although S. indicus extract at high dose showed remarkable efficacy in psoriasis, further studies with longer duration and larger sample size are required to confirm efficacy and safety of S. indicus extract (Tinefcon® ).
5. Conclusions
- S. indicus extract (Tinefcon® ) was safe and well tolerated in both the dosage strengths; however it was more effective in subjects with moderate to severe plaque type psoriasis at the higher dosage of 2.8 g/day.
ACKNOWLEDGEMENTS
- The authors are thankful to Dr. G. Tyebkhan for her expert comments and review of the manuscript, K. Salkar for administrative support and Karmic Lifesciences LLP for drafting of the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML