-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2015; 4(3): 30-36
doi:10.5923/j.ajdv.20150403.02
Isolation, Identification and Antifungal Susceptibility Test of Dermatophytes from the Patients with Onychomycosis in Central Nepal
Jha BK1, Mahadevamurthy S.2, Sudisha J.3, Bora A.1
1Assistant Professor, Department of Microbiology, College of Medical Sciences, Bharatpur, Nepal
2Associate Professor, Department of Microbiology, Yuvaraja’s College, University of Mysore, Mysore, India
3Laboratory of Molecular Plant Pathology, Department of Biological and Environmental Sciences, Faculty of Agriculture, Yamaguchi University, Yamaguchi, Japan
Correspondence to: Jha BK, Assistant Professor, Department of Microbiology, College of Medical Sciences, Bharatpur, Nepal.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Onychomycosis is a most common nail infection mainly caused by dermatophytes, and occasionally by yeasts and non-dermatophytic molds. Trichophyton rubrum, T. mentagrophytes, and Epidermophyton floccosum are the most common etiologic agents worldwide. This research was done to determine the prevalence of onychomycosis by molecular characterisation and antifungal susceptibility test for dermatophytes causing tinea unguium. Samples were collected and processed over a period of three years (Jan 2012 - Dec 2014) samples from 500 patients with clinically suspected fungal nail infections were enrolled for our study. The nail clippings were collected and subjected to microscopy and culture. Culture positive phenotypically confirmed dermatophytes were further characterised by PCR and antifungal susceptibility were done by MIC. The prevalence rate of dermatophytic onychomycosis was higher among 30-50 years age group patients but males had higher infection rate than females. The main etiological agents of tinea ungium were Trichophyton mentagrophytes, T. rubrum, Epidermophyton floccosum, T.tonsurans. All morphologically and biochemically confirmed dermatophytes for onychomycosis were also confirmed by PCR which were found to be in agreement with the conventional phenotypic methods. The range of their MIC endpoint by broth microdilution technique towards the five antifungal drugs was as follows: terbinafine, ketoconazole, itraconazole and griseofulvin from 0.03-4 mcg ml-1 where as for fluconazole from 4-64 mcg ml-1. Terbinafine is the most sensitive drug when compared to other azoles like fluconazole which is slowly moving towards the resistance. Terbinafine is the drug of choice for dermatophytes. Routine PCR and MIC for antifungal agents serves as a rapid and reliable method for isolation, essential for epidemiological purpose and also helpful for treatment, especially in immunocompromised and young children.
Keywords: Dermatophytes, Culture, PCR, Microbroth dilution technique
Cite this paper: Jha BK, Mahadevamurthy S., Sudisha J., Bora A., Isolation, Identification and Antifungal Susceptibility Test of Dermatophytes from the Patients with Onychomycosis in Central Nepal, American Journal of Dermatology and Venereology, Vol. 4 No. 3, 2015, pp. 30-36. doi: 10.5923/j.ajdv.20150403.02.
Article Outline
1. Introduction
- Onychomycosis is defined as a superficial fungal infection of nail plate from finger and toe [1]. The 80-90% of etiological agents of onychomycosis are dermatophytes, mainly by Trichophyton rubrum and Trichophyton mentagrophytes and occasionally by Epidermophyton flocossum [2, 3]. Trichophyton rubrum is found in 56% of cases, T. mentagrophytes in 24% and E. flocossum in 9%. The rest originate from non-dermatophyte mold such as Scopulariopsis spp., Scytalidium spp., Acrimonium spp., Fusarium spp. and Aspergilus spp. (5-11%) [4]. The worldwide incidence of onychomycosis is increasing and a number of factors contribute to this rise [5]. Several studies have been done to access the prevalence of onychomycosis in the community as well as in the urban area to know the causative agents responsible for onychomycosis. There are different factors associated with the increasing dermatophytosis are humidity, contaminated footwear, traumatic nail, genetic factor and in different diseases in which our immunity gets suppressed like diabetes, poor peripheral circulation, immunosuppressive therapy and HIV infection [6]. In developing countries due to low awareness of onychomycosis, it continues to spread and persists in the community and also remains in the form of normal flora. Moreover, nail alterations similar to onychomycosis can be caused by dermatoses such as psoriasis, lichen planus and melanoma, therefore diagnosis and treatment of fungus nail infection is critical [7, 8]. Diagnosis and confirmation of this infection depends on laboratory identification, which is based on direct microscopy and culture of specimens.The molecular characterization and antifungal susceptibility test of onychomycosis has been well-studied in some developed countries, but few data are available in tropical countries. This study, therefore, seeks to improve knowledge of the molecular identification and the antifungal susceptibility test of onychomycosis. The prevalence of onychomycosis has increased tremendously in the last few decades due to various factors like climatic changes socio-economical and occupational situations. Hence, it is necessary to investigate region wise causative agents for onychomycosis and its susceptibility pattern against the antifungal agents. The aims of the present study were to determine the prevalence of onychomycosis, molecular characterisation and antifungal susceptibility test for dermatophytes causing tinea unguium and tinea pedis in the adult population of Mysore as well as the percentage of individuals who presented with both disorders simultaneously.
2. Materials and Methods
- Clinical specimensOver a period of three years (Jan 2012 - Dec 2014) samples from 500 patients with clinically suspected fungal nail infections, who attended dermatology outpatient department at College of Medical Sciences, Bharatpur, Nepal were enrolled for our study. The assessments of the patients were conducted by interview, clinical examination, entry in questionnaire and collection of nail samples for microbiological studies. Patients with other types of dermatophytosis, yeast infection and non-dermatophytic molds infected patients were totally excluded from the present study. Ethical clearance was taken from Institutional Human Ethical Committee, College of Medical Sciences, Bharatpur, Nepal (IHEC/COMS/No. 174/PhD/2011-12. Dated: 02-12-2011). The specimens were collected from infected nails by nail eclipses, scraping or clipping after cleaning with 70% of ethanol. All specimens were analysed as soon as possible by direct microscopy in 20% KOH for fungal element and culture. All samples were inoculated in plain SDA (HiMedia laboratories) and also in a SDA with chloramphenicol, gentamycin and cyclohexamide in duplicate then incubated at 25°C and 37°C than examined daily for six weeks. The onychomycosis causing dermatophytes were identified, based on their colony morphology, microscopic examination in the Lactophenol Cotton Blue (LPCB) preparations from slide culture techniques. The further identification of the dermatophyte species were done, based on the urease production, pigment production on corn meal agar, in-vitro hair perforation test and vitamin utilisation test [9-11].Optimization of PCR directly from Nail sample:The genomic DNA was directly obtained from the nail by using proteinase K. The nail clippings were added with 500μl of buffer along with 30µl of resin and they were ground by using a mortar and pestle. The nail specimens and 15 μl of proteinase K were added and they were incubated overnight at 60°C. After adding 200µl of chloroform, the samples were spun at 5000 rpm for 15 minute. The supernatant was taken than 50µl of DNA stripping solution was added and the vial were incubated at 60°C for 10 min. Hundred µl of precipitated solution was added and the vials were spun at 1500rpm for 5 min. To the supernatant, ice cold isopropyl alcohol was added to precipitate the DNA. Finally, the DNA was washed with 70% ethanol and it was re-suspended with 100µl of Tris EDTA buffer then it was restored at -20°C for reuse [12].Diagnostic sensitivity: The ten-fold serial dilution was done with the T. rubrum ATCC 28188 to estimate the diagnostic sensitivity of the PCR assay. Diagnostic specificity: Two reference strains, T. mentagrophytes (ATCC 9533) and T. verrucosum (ATCC 52066) were used to ascertain the specificity of the dermatophytes specific primer. Laboratory isolated E. coli boiled extract DNA were used to test the specificity of PCR primers as negative control.Pan fungal primers which targeted the ITS regionUniplex PCR was performed by using Trichophyton spp. primers ITS 1 (5'- TCC GTA GGT GAA CCT GCG G-3') and ITS 4 (5'-TCC TCC GCT TAT TGA TAT GC-3') [13]. The primers and the PCR reagents were purchased from Bangalore Genei Pvt. Ltd., India. The PCR was carried out by using the eppendrof master cycler gradient. The PCR reaction mixture constituted of 200µM concentration of each dNTPS, 25 pmol of each primer, 1 U of Taq polymerase, 5µl of buffer and 10 µl of DNA template. Sterile nuclease free water was added to make final volume of 50 µl. The PCR amplification conditions of an initial denaturation at 95°C for 4 min, followed by 35 cycles at 95°C for 30s, 55°C for 1 min and a final extension at 72°C for 4 minute.Detection of amplified products:The PCR amplified products were elecrophoretically separated in a 2% agarose gel in 1× Tris acetate- EDTA buffer and they were visualized by using ethidium bromide, under a UV trans illuminator [13, 14].Antifungal susceptibility test of isolated dermatophytes:Antifungal susceptibility test was done according to the microdilution method for filamentous fungi M38-A [15] of the national committee for clinical laboratory standards (NCCLS 2002) against the five antifungal agents; terbinafine, itraconazole, ketoconazole, fluconazole and griseofulvin [16]. The drugs were obtained from their respective manufacturer's viz., fluconazole (Cipla Pharmaceuticals Ltd., India), ketoconazole, itraconazole (Cadila Pharmaceuticals, India), terbinafine (HiMedia, India) and griseofulvin (HiMedia, India). Fluconazole was dissolved in distilled water while the other drugs were dissolved in 100% dimethyl sulfoxide (Cipla pharmaceuticals Ltd., India). They were subsequently prepared as stock solution and serial two-fold dilutions were performed. Serial dilutions were carried out using antifungal agents like terbinafine (0.03-0.5), itraconazole (0.03-0.25), ketoconazole (0.03-4), fluconazole (4-64) and griseofulvin (0.24-1) µg ml-1.Test procedure:Inoculum suspensions were prepared from the seven days old cultures grown on PDA at 28°C. The fungal colonies were covered with approximately 10 ml of distilled water and the suspensions were made by scraping the surface with the tip of a sterile loop. The resulting mixture of conidia and fragments of hyphae were withdrawn and transferred to sterile tubes and left for 15-20 min at room tem to sediment the heavy particles. The optical density of the suspensions containing conidial and fragments of hyphae was read at 530nm, adjusted to transmittance of 65-70% (2-4 × 106 cells ml-1) and dilution with Roswell park memorial institute (RPMI) 1640 medium (Sigma Co. St. Louis, USA) to obtain the final inoculum size of approximately 0.4-5 × 104 cells ml-1. Aliquots of 100µl of these suspensions were inoculated in well of micro-titre plate containing 100µl of specific antifungal drugs concentration and incubation at 28°C. Each assay was carried out in duplicate [17-19].End point determinations: End point values were performed visually every 24 h until the indication of growth in drug free control well. For azole agents and griseofulvin, the MIC was defined as the lowest concentration that produced prominent inhibition of growth (approximately 80% inhibition), while terbinafine was defined as lowest concentration showing 100% growth inhibition [20-21].
3. Results
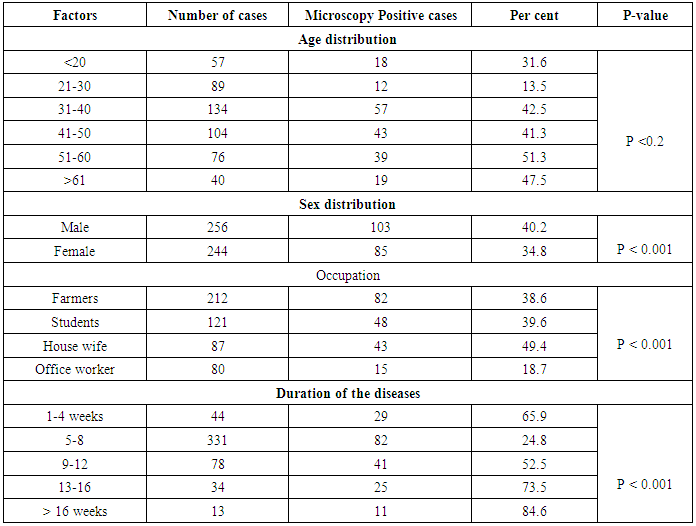
- A total of 500 patients were examined, among them 256 were males and 244 were females. Out of 500 patients with clinical lesion in the nail 188 (37.6%) had dermatophytic onychomycosis by direct microscopy and 231 (46.2%) culture positive. Clinical specimen from 43 patients were positive in culture did not show fungal element in the microscopic examination. The prevalence rate of dermatophytic onychomycosis was higher among 30-50 years age group patients but males 103 (40.2%) had higher infection rate than females 85 (34.8%). Farmers and students were found to have a higher rate of onychomycosis, and it was also found to be persistent for longer period without any ill effect of nail, so most of the patients visited to the doctor in 5-8 weeks after the infection (Table 1).
|
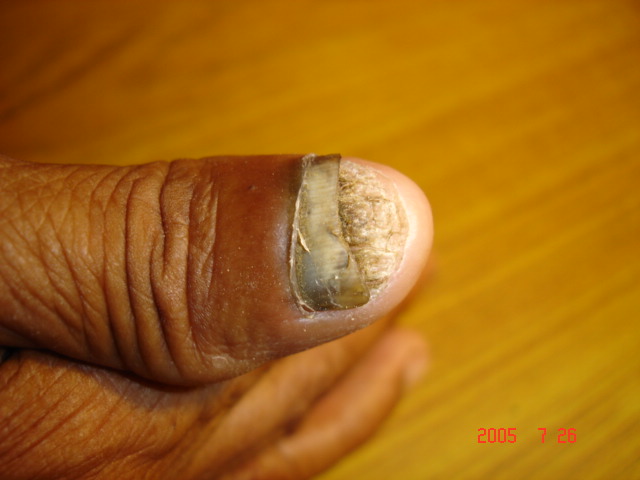 | Figure 1. Distal subungual hyperkeratosis with nail dystrophy |
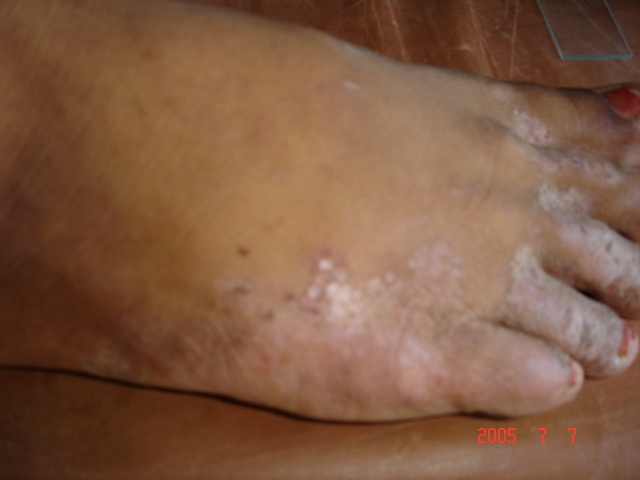 | Figure 2. Vesicular form (vesiculo-pustules on dorsum surface of feet and nail infection) |
|
|
4. Discussion
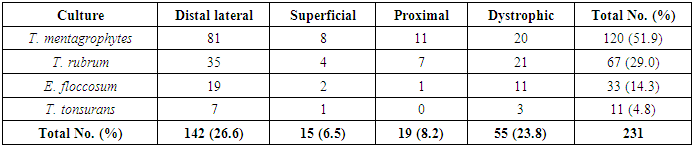
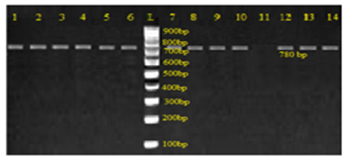
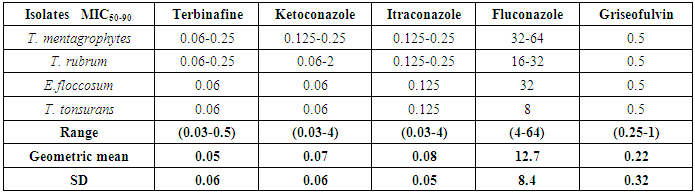
- With the increasing incidence of antifungal resistance pattern of dermatophytic onychomycosis, the requirement for proper isolation, identification and antifungal susceptibility test is very much necessary. In addition, the traditional microscopy and culture methods are slow and non-specific diagnostic approaches, and hence molecular tools for fungal identification of dermatophytes are considered as great help [1, 22].In our study out of 500 patients were examined, among them 256 were males and 244 were females. Onychomycosis was found to affect all age groups. Out of 500 patients with clinical lesion in the nail 188 (37.6%) were found to be infected with dermatophytic onychomycosis by direct microscopy and 231 (46.2%) were culture positive. In another research there were mixed type of report found about onychomycosis, because disease remains asymptomatic and some patients were taking over the counter medication [23]. Clinical specimens from 43 patients were shown to be culture positive but did not show fungal element in the microscopic examination. The prevalence rate of dermatophytic onychomycosis was higher among 30-50 years age group patients but males 103 (40.2%) had higher infection rate than females 85 (34.8%). Present result has been observed in agreement with the result obtained by most of the researchers from India and abroad [18, 24]. Farmers and students were found to have a high rate of onychomycosis, and it was persistent for longer period without any ill effect of nail, so most of the patients visited doctor after 5-8 weeks of infection (Table 1). Raberts (1992) found, person’s burden of wet work and increased trauma facilitating easy entry of dermatophytes in the nail of figure and toe [25].In this study the most common clinical presentation observed in all type of onychomycosis was nail discoloration (100%) and subungual hyperkeratosis (90%). The clinical features of the nail affected by tinea ungium were nail lyses, hyperkeratosis and nail discoloration as shown in Figs. 1 and 2. Hay (1993) found, most of the house wives were having more rates of finger nail onychomycosis than toe nail [26]. In present study the nail most commonly affected was toe nail and least affected was finger nails among females. One hundred and forty two (26.6%) patients were presented with distal lateral onychomycosis, 55 (23.8%) were having total dystrophic, 19 (8.2%) proximal and 15 (6.5%) with superficial onychomycosis.Sabourauds Dextrose Agar (SDA), Dermatophytes Test Medium (DTM) and Bromocresol Purple Agar (BCP) were used for selective culture of only dermatophytes by incubation at 28oC for 2-4 weeks [5-7, 9]. In previous studies, it was found that the rate of isolation of T. mentagrophytes and T. rubrum was very high which can be explained on the basis of its greater capacity to infect the hard keratin of the nail [27]. The main etiological agents of tinea ungium were Trichophyton mentagrophytes 120 (51.9%), T. rubrum 67 (29.0%), Epidermophyton floccosum 33 (14.3%), T. tonsurans 11 (4.8%) (Table 2). Epidermophyton floccosum 33 (14.3%) and T. tonsurans 11 (4.8%) is the second most common isolated dermatophytes in our study which were isolated from both finger and toe nail onychomycosis. Out of 142 cases of distal lateral onychomycosis 81(28) were shown positive towards T. mentagrophytes and second highest was T. rubrum where in 35 cases were shown to be culture positive. English (1976) found that T. mentagrophytes and T. rubrum are the most common dermatophytes in the aetiology of the distal lateral onychomycosis [29]. Non dermatophytic growths were also positive but they were totally excluded from the present study.The traditional, culture-based methods of identifying dermatophytes are cumbersome, laborious, time consuming and often inconclusive, due to fungal phenotypic variability and pleomorphism [1, 30]. However, the advancement of molecular biology has new molecular tool to the diagnosis of dermatophytic infections. One such approach, introduced by Howell et al., (1993) relies upon PCR amplified ITS regions of the rDNA gene complex [31]. The disagreement between the two methods of identification was reported by Gupta and Kohli (2003) [32]. The results from the present study showed high concordance between conventional and molecular techniques for identifying Trichophyton spp. and Epidermophyton spp. All morphologically and biochemically confirmed dermatophytes for onychomycosis were genotypically also confirmed and found agreement with the conventional phenotypic methods with 100% sensitivity and specificity. Out of 231 phenotypically confirmed cases all showed genotypically positive by PCR (Figs. 3 and 4).In the present study, the NCCLS M38-A broth microdilution method was used to determine the antifungal susceptibility pattern of dermatophytes isolated from the clinical specimens. Table 3 shows the pattern of antifungal susceptibility of the dermatophytes causing onychomycosis and the range of their MIC endpoint by broth microdilution technique (NCCLS M38-A) toward the five antifungal drugs. The MIC ranges, mean and standard deviation (SD) were as follows: for terbinafine from 0.03-0.5 (0.05, 0.06) mcg ml-1, ketoconazole from 0.03-4 (0.07, 0.06) mcg ml-1, itraconazole ranged from 0.03-4 (0.08, 0.05) mcg ml-1, fluconazole from 4-64 (12.7, 8.4) and griseofulvin from 0.25-1(0.22, 0.32) mcg ml-1. Our results were significantly higher than the result obtained by Barros and Hamdon (2005) where MICs were, 0.007-0.0015 for terbinafine, 0.062-1.0 for itraconazole, 0.025-2.0 for griseofulvin and 0.125-2 for ketoconazole. Our reports were also higher than the MIC ranges, mean and SD obtained by Brilhalte et al. (2005), Pujol et al. (2005) and Esteban et al., (2005) [33-35]. The higher MIC than those of other research, possibly because of increased use and misuse of local oral antifungal agents, partial dose of the drug for shorter period and over the counter medication directly by patient remains chronic infection. Terbinafine is the most sensitive drug against dermatophytes, it provides long term clinical efficacy and lower relapse as mentioned by Darkes et al. 2003 [36]. When compared to other azole which is slowly moving towards the resistance, fluconazole is the drug which is more frequently used in the hospital and over the counter medication in the community so it is acquiring resistance. Hence, this was in accordance with the results obtained by other researchers [37, 38].
5. Conclusions
- This is the first report of molecular characterization and in vitro antifungal sensitivity test for onychomycosis causing dermatophytes and non-dermatophytic moulds in Nepal. T. mentagrophytes and T. rubrum were most common isolates. Genotypic differentiation by PCR provides a rapid and practical tool for identification of dermatophytes with precision and accuracy. Although the onychomycosis is not a life threatening, it can be source of pain and discomfort; it can easily transmit disease from one to another person. Invasive and long term use of antifungal leads to a decline in sensitivity and resistant development of dermatophytes. Antifungal susceptibility test by broth microdilution technique is method of choice where disc diffusion is not applicable. Terbinafine is the drug of choice for dermatophytes. Even it is not a lethal disease it can generate physical, psychological and occupational problems considerably impairing patients quality of life. Routine MIC for antifungal agents is essential for epidemiological purposes and also useful for immunocompromised patients and young children. Therefore, we believe that species confirmation and antifungal sensitivity test will be very useful in managing treatment and preventing resistant development.
ACKNOWLEDGEMENTS
- The authors like to thank Department of Dermatology staff, College of Medical Sciences, Bharatpur, Nepal for continuous support and encouragement.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML