-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2014; 3(3): 57-62
doi:10.5923/j.ajdv.20140303.02
Topical Therapy of Psoriasis Using Zinc Sulphate Cream 5% and 10%
Khalifa E. Sharqui1, Adil A. Noaimi2, Ali R. Auda3, Wesal K. Al-Janabi4
1Scientific Council of Dermatology and Venereology – Iraqi Board for Medical Specializations, Department of Dermatology & Venereology, College of Medicine, University of Baghdad, Baghdad, Iraq
2Head of Department of Dermatology and Venereology, College of Medicine, University of Baghdad, Baghdad, Iraq
3Department of Dermatology and Venereology, Baghdad Teaching Hospital, Baghdad, Iraq
4Department of Dermatology and Venereology, Baghdad Teaching Hospital, Medical City, Baghdad, Iraq
Correspondence to: Khalifa E. Sharqui, Scientific Council of Dermatology and Venereology – Iraqi Board for Medical Specializations, Department of Dermatology & Venereology, College of Medicine, University of Baghdad, Baghdad, Iraq.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background: Psoriasis is an immunologically mediated disease that associated with a reduced zinc level in plaques and serum. Objective:To evaluate the effectiveness of topical zinc sulphate cream 5% and 10% in the treatment of psoriasis in comparison with aqua Rosa cream as a control group. Patients and Methods: This single blind placebo controlled study was conducted in Department of Dermatology – Baghdad Teaching Hospital, during the period from April 2007 to October 2008. A total of 56 patients with mild to moderate plaque psoriasis were enrolled in this study. There were 32 (57.1%) males and 24 (42.9%) females with a male to female ratio of 1.3:1. Their ages at presentation ranged from 4-72(31.06 ± 16.77) years. The patients were divided into two groups: 28 patients in Group A were treated with zinc sulphate cream {13 patients were treated with 5% (part 1) and 15 patients were treated with 10% (part 2)} and 28 patients in Group B were treated with aqua Rosa cream as a control. All patients were treated with twice daily regimen. PASI score was assessed for every patient at each visit. The patients were seen regularly every 2 weeks for 12 weeks during the treatment period and then for another 4 weeks after cessation of treatment in those patients who achieved good response. Results: The percentage of patients who achieved good response to treatment was as follows: Group A (part 1) 7 (53.8%) patients, Group A (part 2) 9 (60%) patients While in Group B 2 (7.1%) patients had good response. Four weeks after the cessation of treatment the relapse rates were 6 (85.7%) patients in Group A (part 1) and 7 (77.8%) patients in Group A (part 2). While in Group B, 2 (100%) patients relapsed during the first 2 weeks after cessation of the treatment. Conclusion: Zinc sulphate cream 5and 10% had proved to be an effective safe treatment of psoriasis as it achieved good response in 53.8%-60% of patients respectively with relatively few side effects.
Keywords: Topical Zinc Sulphate Cream 5% and 10%, Psoriasis
Cite this paper: Khalifa E. Sharqui, Adil A. Noaimi, Ali R. Auda, Wesal K. Al-Janabi, Topical Therapy of Psoriasis Using Zinc Sulphate Cream 5% and 10%, American Journal of Dermatology and Venereology, Vol. 3 No. 3, 2014, pp. 57-62. doi: 10.5923/j.ajdv.20140303.02.
1. Introduction
- Psoriasis is a common, chronic, disfiguring, inflammatory and proliferative condition of the skin, in which both genetic and environmental influences have a critical role in its aetiopathogenesis. [1] Now, psoriasis is considered as an immunologically mediated disease towards not fully defined antigens [2]. Despite the importance of systemic therapies and the advances represented by biologics, topical treatment will probably remain the mainstay of psoriasis therapy especially for patients with mild type [3]. Zinc is one of the essential trace elements [4] found in every cell in the body where more than 300 enzymes in the body need zinc in order to function properly [5]. Zinc has successfully been used in the treatment of many skin diseases such as cutaneous leishmaniasis [6-8] viral warts [9-11] and recurrent oral aphthosis and in Behcet’s disease [12] where it was found to be safe and effective therapy.The mechanism of action of zinc sulphate include the followings:* Immunological action as immunomodulator, zinc sulphate may actually cause suppression of unwanted immune reactions [13, 14]. Patients with active psoriasis vulgaris had a significant increase in neutrophil random migration and directed chemotaxis. Zinc sulphate treatment restores both the random migration and directed chemotaxis to normal values (zinc sulphate modifies neutrophil inflammatory potential) [15]. Also zinc sulphate corrects the imbalance between Th1 and Th2 cytokines [16]. * Anti-inflammatory effect through inhibition of prostaglandins [17].* Anti-proliferative mechanism of action may involve the regulation of DNA transcription factors (containing zinc finger binding domains) [18, 19].* It is also well known that a deficiency of zinc produce a disease state (acrodermatitis enteropathica) that include psoriasiform lesion [20, 21].* Anti-oxidant activity: reactive oxygen species have been implicated in the pathogenesis of various hyperproliferative and inflammatory disease. Studies showed that the expression of the antioxidant enzyme superoxide dismutase was consistently higher in lesional psoriatic skin as compared to uninvolved skin [22, 23].* On high concentration, zinc has a direct cytotoxic effect and is well known to induce apoptosis and tissue necrosis [22].Therefore the present study was designed to evaluate the effectiveness of topical zinc sulphate cream 5% and 10%, in the treatment of psoriasis in comparison with aqua rosa cream as a control group.
2. Patients and Methods
- This single-blind placebo controlled study was conducted in the Department of Dermatology and Venereology, Baghdad Teaching Hospital, during the period from April 2007 to October 2008. The nature and target of this study were explained for each patient. Formal consent was taken from each patient before starting the therapy, after full explanation about the nature of the disease, course, and the options of treatment, follow up, prognosis, complications and the need for pre and post treatment photographs. Also, the ethical approval was given by the scientific committee of the Scientific Council of Dermatology & Venereology-Iraqi Board for Medical Specializations.A total of 70 patients with mild and moderate plaque psoriasis were enrolled in this study.The diagnosis was established on clinical basis. Patients with prior treatment in the last 2 weeks and immunosuppressed patients were excluded from the study.History was taken regarding: gender, age, occupation, residence, age of onset, duration of the disease, presence of itching, history of previous treatment and personal or family history of psoriasis. Physical examination was done to assess the site, surface area and severity of psoriasis using PASI score. Pre and post treatment photographs were taken using Sony-digital, high sensitivity, 6 mega pixels, Dsc-50 still camera, in the same place with fixed illumination and distance.The treated patients were divided into 2 groups:Zinc sulphate group (Group A): This was subdivided into 2 parts: Part 1: 16 patients treated by zinc sulphate cream 5% and three Patients defaulted during the course of therapy. Part 2: 17 patients treated by zinc sulphate cream 10% and two Patients defaulted.Control group (Group B): 37 controlled patients treated by aqua Rosa cream as a control. Nine patients defaulted at different times of therapy.All patients in the 2 groups were treated with twice daily regimen.The patients were seen regularly every 2 weeks for 12 weeks then for another 4 weeks after cessation of treatment in those patients who achieved good response with a reduction in PASI score > 50%. At each visit the response to treatment was assessed according to the reduction in PASI score. Any sign or symptom of side effects of treatment modalities was reported.PASI score was calculated as follows: The psoriatic plaques were graded based on three criteria (index): Redness, thickness, and scaling each was assigned as a number from 0 to 4 with 4 being the worst. The three index scores are added up for each of the four body regions to give subtotals A1, A2, A3, and A4. Each subtotal is multiplied by the body surface area represented by that region. In each of these areas, the fraction of total surface area affected is graded on a 0-6 scale (zero for no involvement; up to 6 for greater than 90% involvement). Then each of the body area scores is multiplied by the area affected. The PASI score is the summation of scores of all affected areas.Assessment of drug efficacy was based on the reduction of PASI score. Patients were considered as good responders if the reduction in PASI score is 50% or more [24, 25].Relapse (only for patients who achieved at least 50% improvement in PASI score from baseline) occurs when the improvement in the PASI score falls below 50% from the baseline PASI score [13].To prepare 5% zinc sulphate cream; 5 grams of zinc sulphate crystal (ZnSO4 7H2O = 287.54 from MERK, France) was mixed in liquid paraffin (10 ml) and then mixed up to 100 grams Aqua Rosa.To prepare 10% zinc sulphate cream, 10 grams of zinc sulphate crystal (ZnSO4 7H2O = 287.54 from MERK, France) was mixed in liquid paraffin (20 ml) and then mixed up to 100 grams Aqua Rosa.For the determination of statistical significance among different variable, descriptive statistics (mean and standard deviation) were used together with analytic statistics (chi-square, t-test and ANOVA) using EPI-Info version 6.
3. Results
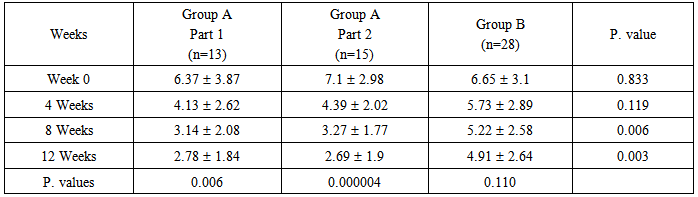
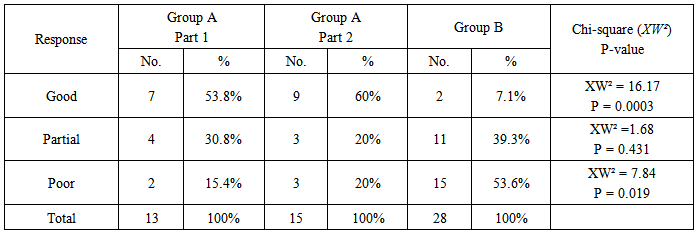
- A total of 70 patients were enrolled in this study, 14 of them were defaulted from the study at different weeks for unknown reason and their results were omitted. The remaining patients 32 (57.1%) males and 24 (42.9%) females with a male to female ratio 1.3:1, their ages at presentation ranged from 4-72 years with a mean31.06 ± 16.77 years; their ages at onset ranged from 1-61 years with a mean 24.83 ± 13.5 years. The disease duration ranged from 2 weeks to 30 years with a median 4 years. Itching was reported in 45 (80.4%) patients which varied in severity from mild to severe.Family history of psoriasis was positive in 19 (33.9%) patients. Nail changes were found in 19 (33.9%) patients.Group A(Part 1): 13 patients, of these patients 7 (53.8%) males and 6 (46.2%) females with a male to female ratio 1.17:1; their ages at presentation ranged from 6-72 years with a mean31.09 ± 19.09 years; their ages of onset ranged from 4-61 years with a mean26.13 ± 14.97 years. The disease duration ranged from 2 weeks to 15 years with a median 2.5 year. Itching was reported in 10 (76.9%) patients, which varied in severity from mild to severe. Family history of psoriasis was positive in 4 (30.8%) patients. Nail changes were found in 5 (38.5%) patients.The mean PASI score at the baseline was 6.37 3.87 changed to 2.78 1.84 after 12 weeks of treatment, this reduction in PASI score was statistically significant with P-value= 0.006 (Table-1).The response to treatment was divided into (Table-2):a. Good response was achieved in 7 (53.8%) patients with a reduction in PASI score > 50%.b. Partial response was achieved in 4 (30.8%) patients with a reduction in PASI score ranged from 25-49%.c. Poor response was achieved in 2 (15.4%) patients with a reduction in PASI score < 25%.Regarding the side effects; there was a transient, mild burning sensation reported in 4 (30.76%) patients at the initial time of application which disappeared after few minutes and no other side effects were detected.Group A (Part 2): 15 patients of these patients 9 (60%) males and 6 (40%) females with a male to female ratio 1.5:1; their ages at presentation ranged from 4-61 years with a mean32.30 16.51 years; their ages of onset ranged from 1-51 years with a mean24.96 13.68 years. The disease duration ranged from 6 months to 30 years with a median 4 years. Itching was reported in 13 (86.7%) patients which varied in severity from mild to severe. Family history of psoriasis was positive in 6 (40%) patients. Nail changes were found in 4 (26.7%) patients. The mean PASI score at the baseline was 7.1 2.98 reduced to 2.69 1.9 after 12 weeks of treatment, this reduction in PASI score was statistically highly significant with P-value= 0.000004 (Table-1).The response to treatment was divided into (Table- 2):a. Good response was achieved in 9 (60%) patients with a reduction in PASI score > 50%.b. Partial response was achieved in 3 (20%) patients with a reduction in PASI score ranged from 25-49%.c. Poor response was achieved in 3 (20%) patients with a reduction in PASI score was < 25%. Regarding the side effects; there was a transient, mild burning sensation reported in 6 (40%) patients at the initial time of application which disappeared after few minutes and no other side effects were detected.Group B: 28 patients of these patients 16 (57.1%) males and 12 (42.9%) females with a male to female ratio 1.3:1; their ages at presentation ranged from 7-62 years with a mean29.8 14.73 years; their ages of onset ranged from 5-60 year with a mean 23.42 11.85 years. The disease duration ranged from 3 months to 25 years with a median 3.5 years. Itching was reported in 22 (78.6%) patients which varied in severity from mild to severe. Family history of psoriasis was positive in 9 (32.1%) patients. Nail changes were found in 10 (35.7%) patients. The mean PASI score at the baseline was 6.65 3.1 reduced to 4.91 2.64 after 12 weeks of treatment, this reduction in PASI score was statistically not significant with P-value = 0.110 (Table-1).The response to treatment was divided into (Table-2):a. Good response was achieved in 2 (7.1%) patients with a reduction in PASI score > 50%.b. Partial response was achieved in 11 (39.3%) patients with a reduction in PASI score ranged from 25-49%.c. Poor response was achieved in 15 (53.6%) patients with a reduction in PASI score < 25%. No side effects were detected.In Group A (part 1); the mean reduction in PASI score was 35.2% after 4 weeks of treatment, 50.7% after 8 weeks of treatment and 56.4% after 12 weeks of treatment.In group A (part 2); the mean reduction in PASI score was 38.2% after 4 weeks of treatment, 53.95% after 8 weeks of treatment and 62.1% after 12 weeks of treatment.In Group B, the mean reduction in PASI score was 13.8% after 4 weeks of treatment, 21.5% after 8 weeks of treatment and 26.2% after 12 weeks of treatment.The difference in the degree of improvement (reduction in the PASI score) between zinc sulphate groups (5% vs. 10 %) after 12 weeks of treatment were statistically not significant with P-value = 0.989 (Table-1).However, the difference in the degree of improvement (reduction in the PASI score) between zinc sulphate groups and aqua rosa group after 12 weeks of treatment were statistically significant with P-value = 0.003 (Table-1).The relapse rate in patients with good response:In Group A (part 1), 4 (57.1%) patients relapsed during the first 2 weeks after cessation of treatment and 2 (28.6%) patients relapsed after 4 weeks of stopping the treatment. The relapse rate was found in 85.7% of cases. One (14.3%) patient didn't relapse.In Group A (part 2), 4 (44.5) patients relapsed during the first 2 weeks after cessation of treatment and 3 (33.3%) patients relapsed after 4 weeks of stopping treatment. The relapse rate was found in 77.8% of cases. Two (22.2%) patients didn't relapse.In Group B, 2 (100%) patients relapsed during the first 2 weeks after cessation of treatment. So, the relapse rate was 100% because in this group only 2 patients had good response and these 2 patients relapsed after stopping therapy. Accordingly the relapse rate was 100%.
|
|
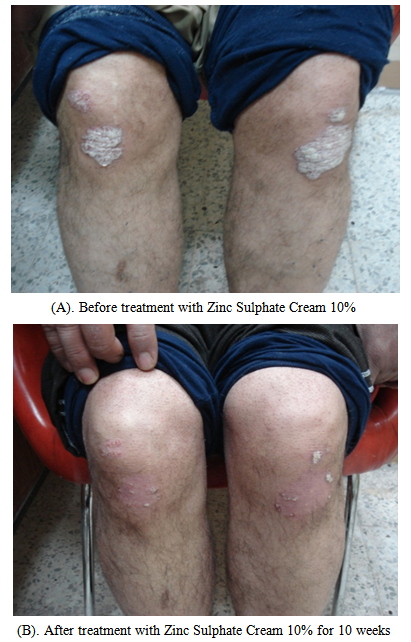 | Figure 1. Forty two years old male with plaque psoriasis |
4. Discussion
- Psoriasis is thought to be an immunologically mediated disease where T-cell plays an important role in its pathogenesis [3]. Till now unfortunately, there is no unique curative systemic or topical treatment.Zinc sulphate has been used as immunomodulator in the treatment of many dermatological problems such as alopecia areata [26, 27], cutaneous leishmaniasis [8], rosacea [28], erythema nodosum leprosum [29], Behcet's disease [12], and recalcitrant common warts [9, 11]. Yet trial of oral zinc sulphate proved to be ineffective in treatment of psoriasis [15].Recent reports found that zinc level is low in plaques and serum of psoriatic patients [30.19]. Accordingly, zinc sulphate has been used in this study as a topical treatment.The present study showed that zinc sulphate cream 5% and 10%achieved good response in 53.8% and 60% of patients after 12 weeks of treatment respectively but the onset of action seemed to be slow as the patients started to notice improvement after 8 weeks of treatment with each concentration of cream, while topical application of aqua rosa cream achieved good response in about 7.1% of patients after 12 weeks of treatment. The difference in the degree of improvement (reduction in PASI score) between zinc sulphate groups (5% vs. 10%) after 12 weeks of treatment were statistically not significant with p-value = 0.989. Accordingly we recommend using zinc sulphate cream 5%rather than 10%. On the other hand, the difference in the degree of improvement between zinc sulphate groups and aqua rosa group after 12 weeks of treatment were statistically significant with p-value = 0.003. The present study also showed that 85.7% and 77.8% of those patients who achieved good response with zinc sulphate cream 5% and 10% respectively relapsed 4 weeks after cessation of treatment while 100% of those treated with aqua Rosa cream relapsed during the first two weeks of treatment. This does indicate that the three groups of topical treatment that had been used in this study didn't affect the relapse rate of the disease.Regarding the topical application of aqua Rosa in Group B the improvement could be explained as follow: it is well known that emollient has semi-occlusive effect against psoriasis. In addition, the application of aqua Rosa might gave psychological relive giving placebo effect. In comparison with a previous uncontrolled Iraqi study [31], zinc sulphate cream 2.5% had achieved good response in about 53.3% of patients after 12 weeks of treatment; which was comparable with the present work where zinc sulphate cream 5%-10% had achieved good response in 53.8%-60% of patients after 12 weeks of treatment [31].The relapse rates in the present research were 85.7% and 77.7% which were comparable to the relapse rate in the previous Iraqi study (75%) [31].The results of the present work showed that the rate of good response achieved by zinc sulphate cream 5%-10% was comparable to that produced by the calcipotriol ointment (53.8%-60% vs. 53.8%) [32].The present study showed that zinc sulphate cream was effective in all age groups while some studies showed that the efficacy of calcipotriol ointment in childhood was similar to placebo [33].In addition zinc sulphate cream is cost effective therapy in comparison with calcipotriol ointment.The study also showed that zinc sulphate cream 5%-10% was a safe topical therapy. This does suggest that zinc sulphate cream is superior and safer to the other modalities in the treatment of psoriasis like calcipotriol ointment, as treatment of psoriasis with calcipotriol ointment may be associated with severe hypercalcemia and hypernatremia especially if used over a wide area [34], other adverse effects of calcipotriol ointment included lesional or perilesional irritation and exacerbation of psoriasis [33].In conclusion, zinc sulphate cream is safe moderately effective topical therapy for psoriasis.
Disclosure
- This study is an independent study and not funded by any drug company.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML