-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2014; 3(2): 30-34
doi:10.5923/j.ajdv.20140302.02
Nail Changes in Hemodialysis Patients and Renal Transplant Recipients (A Case-Control Study)
Haider R. Al-Hamamy , Sabeeh A. Al-Mashhadani , Basman M. Fadheel , Saad H. Salman
Department of Dermatology and Venereolgy, College of Medicine, University of Baghdad
Correspondence to: Basman M. Fadheel , Department of Dermatology and Venereolgy, College of Medicine, University of Baghdad.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background: Nail changes are important tools for diagnosis and monitoring progression of many systemic diseases. In chronic renal failure; those on hemodialysis and renal transplant recipients may exhibit various nail changes. Giving a great clue for the renal function status. Objective: To determine, The frequencys of nail changes in patients with hemodialysis and renal transplant recipients, and to investigate whether these nail pathologies were related to hemodialysis and renal transplantation. Methods: The study is an observational cross sectional case control multicenteric study and was carried out at Baghdad Teaching Hospital, AL-Karama Teaching Hospital, and AL-Yarmouk Teaching Hospital in a period extending from September 2009 to December 2010. Two hundred and ten patients undergo hemodialysis and 200 renal transplant recipients were screened for the presence of nail changes. The findings in these groups were compared with findings in 200 healthy individuals. Results: One hundred and fifty three patients (78.85%) in group of hemodialysis and 118 patients (59%) in the renal transplant recipients had at least one type of nail changes. Absence of lunula, half-and-half nails and leukonychia were significantly more common in the patients with hemodialysis and in the renal transplant recipients. Leukonychia was significantly more frequent in the renal transplant recipients than in the hemodialysis patients and controls. Conclusion: Patients undergo Hemodialysis and renal transplant recipients had higher rates of nail changes than the healthy population. Renal transplantation may reduce the frequencies of nail changes; absence of lunula, and half and-half nails. Interestingly, leukonychia increase significantly after renal transplantation.
Keywords: Nail changes, Renal transplant, Hemodialysis
Cite this paper: Haider R. Al-Hamamy , Sabeeh A. Al-Mashhadani , Basman M. Fadheel , Saad H. Salman , Nail Changes in Hemodialysis Patients and Renal Transplant Recipients (A Case-Control Study), American Journal of Dermatology and Venereology, Vol. 3 No. 2, 2014, pp. 30-34. doi: 10.5923/j.ajdv.20140302.02.
1. Introduction
- The nails are flattened elastic structures of a horny texture, placed upon a distal dorsal surface of the fingers and toes. Most of the nail apparatus developed in utero from the primitive epidermis [1]. Therefore it is similar, in many aspects, to the hair follicle and epidermis [2, 3].The main function of the nail apparatus is to produce a strong, relatively inflexible, keratinous nail plate over the dorsal surface of the end of each digit. The nail plate act as a protective covering for the fingertip. By exerting counter pressure over the volar skin and pulp, the flat nail plate adds to the precision and delicacy of both the ability to pick up small objects and many other subtle finger function [4, 5].The cutaneous manifestations of renal disease are primarily encountered in patient with chronic renal failure (C.R.F.) .In contrast, only two skin changes -edema and uremic frost –occur in acute renal failure. Edema is particular feature of acute renal failure with nephrotic syndrome. Uremic frost results from eccrine deposition of urea crystals on the skin surface of individuals with severe uremia. It is now rare because of the wide availability of acute HD [6].Chronic kidney disease is define as kidney damage or glomerular filtration rate <60 ml/mint for 3 months or more, irrespective of the cause. Most patients with chronic kidney disease stages 3-5 progress relentlessly to End-stage renal disease [7]. CRF; refers to an irreversible deterioration in renal function which classically develops over a period of years. Initially It is manifest only as a biochemical abnormality. Eventually, loss of the excretory, metabolic and endocrine function of the kidney leads to the development of the clinical symptoms and signs of renal failure, which referred to as URAEMIA. When death is likely without renal replacement therapy, it is called end stage renal disease (E.S.R.D.) [8].Nail alterations in uraemic patients are common and may affect 71.4% of patients [9]. Half-and half nails [10], absence of lunula [11] and splinter haemorrhage [12] are the most common nail disorders among uraemic patients.The prevalence of nail pathology in the R.T.Rs. increased with age and with longer duration of immunosuppression, but was not influenced by different immunosuppressive treatment protocols [13]. Leukonychia are the most common nail pathology in R.T.Rs. group [13].Absence of lunula was the second most common nail change in the renal transplant recipient group [13].The significantly increased rate of onychomycosis among R.T.Rs. which could be explained on the basis of decreased cell-mediated immunity related to immunosuppressive therapy used to prevent graft rejection [14].The aim of this study was to determine the frequency of nail changes in hemodialysis patients and renal transplant recipients, and to investigate whether these nail changes are related to hemodialysis and renal transplantation.
2. Patients and Methods
- The study is an observational cross sectional case control multicenteric study and was carried out at Baghdad Teaching Hospital, AL-Karama Teaching Hospital and AL-Yarmouk Teaching Hospital in the period extending from September 2009 to December 2010.Nail changes were studied in 410 patients; 210 patients had CRF undergo regular haemodialysis (HD) and 200 patients who had renal transplants (RT). In addition 200 healthy volunteers were also included. Patients on HD were assigned as group A and included 138 males 72 females. Their ages ranged from 17 to 78 years with a mean± SD of 47.97 ±13.77 years (Table1). The duration of HD ranges from 3 to 216 months with a mean± SD of 25.98 ± 27.91 months and the median of 24 months.Group B included 200 patients who had RT; 162 males and 38 females. Their ages range from 14 to 67 years with a mean± SD of 40.86 ±11.63 years. The duration of RT ranges from 3 to 324 months with a mean± SD of 77.29 ±70.2 months and a median of 60 months (Table 1).Group C included two hundred healthy volunteers; 142 males and58 females. Their ages range from 22-68 years with a mean± SD of 46.27 ±12.29 years. They were recruited from the dermatology outpatient department. Some of them were patients with minor dermatological complaint which did not affect the nails while others were relatives accompanying patients to the department.A complete history including age, sex, duration of hemodialysis, frequencies of dialysis per week for HD patients, time of renal transplant for Rt patients, drug history and complete physical examination and investigation where relevant. The finger nails of both hands of all patients were examined carefully with a magnifying lens and any nail change was noted changes in surface, color, thickness, and curvature of the nail plates were recorded. When onychomycosis was suspected, nail clippings were collected for microscopic examination with 10% potassium hydroxide solution. Changes were recorded and Photographs were taken for patients by SONYTM Cyber-shot digital camera DSC- W55 (China).Formal consent was taken from each patient after full explanation about nature of present study and the ethical approval was obtained from the Scientific Council of Dermatology & Venereology - Iraqi Board for Medical Specializations.Statistical analysisAll statistical analyses were performed using the statistical package SPSS for Windows version 10.0. Fisher’s exact chi-square test, one-way ANOVA, and the Student t-test were used to assess statistical relationships between dependent and independent variables. Statistical differences between frequencies of nail disorders in the groups were assessed using Fisher’s exact test. P values >.05 were considered to indicate statistical significance.
3. Results
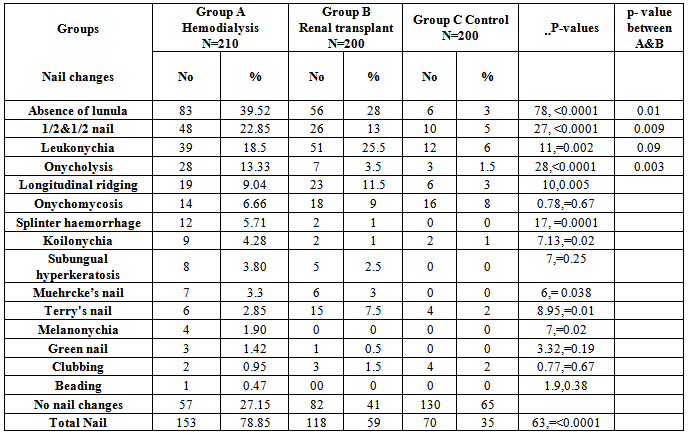
- There was no significant differences among the 3 groups regarding age and gender (p value = 0.18 and 0.38 respectively) (Table 1).
|
4. Discussion
- Although the nail looks a small structure, can be considered as a mirror reflecting pathological changes that occur inside the body. Hence thorough studies are needed to determine the frequencies of the nail changes in different forms of systemic diseases. These changes will act as noninvasive diagnostic and prognostic indicators [1].Renal disease is associated with profound metabolic disturbances that may lead to several changes in the blood supply, innervations and metabolism of growing structures. The nail is easily examined growing structure, therefore the study of nail changes could be one of the indicator for the status of renal function [1].In the present study patients on HD and those with RT had significantly higher numbers of nail changes than the controls. On comparing the percentage of nail changes in patients on HD in the present study with studies from other countries, the present work find that 78.9% of patients on HD had at least one change. This figure is high when compared to results of Tercedor et al in Spain in 2003 [15] who found that 71% of patients on HD had nail changes, while Saray et al showed that 69.8% of their HD patients had nail changes in a study conducted in Turkey in 2004 [13]. The higher number of patients with nail changes in present study could be attributed to two factors; in the present study even minor changes in the nail color, shape & structure were recorded and in the other studies not all changes are recorded which could raise the number of patients with nail changes. The other factor could be related the less efficacy of HD in our Patients; As they received less sessions per week & for a shorter time than what is recommended because of the shortage of HD machines.On comparing the results of RT patients in the present study with that of Saray et al in Turkey in 2004, the results are comparable [13].Concerning RT recipients, they had overall less nail changes than HD patient and this could be due to better renal function in RT patients.The most common 3 nail changes in present study were absence lunula, half and half nail and leukonychia. These are similar to what was previously reported literature however not in the same order. The most common nail change In the present study is absence of lunula while in the study carried out by of Saray et al it was leukonychia [13].The mechanisms by which nail changes occur in renal disease are not fully understood. However different pathomechanisms are suggested. The absence of lunula usually reflects metabolic changes in chronic renal failure and alteration in nail consistency. Iorizzo et al stated that malnutrition, peripheral circulatory diseases, and low level of iron and zinc may be aetiological factors [16]. In addition we suggest that absence of lunula may be correlated to retraction of the nail matrix due to decrease in the nail matrix growth and alteration of hydration of the nail plate make it invisible or due to the attachment to the nail bed.The pathogenesis of half-and-half nails is still unknown. In chronic renal disease the number of capillaries under the nail plate is increased, with remarkable thickening of the capillary walls. The increase in capillary density of the nail bed might account for the band of discoloration [17]. Stimulation of nail melanocytes by increased levels of plasma melanotrophic hormone has been suggested. Substantially higher levels of circulating melanotrophic hormone have been found in patients treated by maintenance dialysis for chronic renal failure [18]. A melanin granule was found in the basal layer of the nail bed epidermis [19]. Regarding leukonychia the changes in the nail bed, are responsible for the white appearance [20]. Nail bed pallor may be a non-specific sign of anemia, oedema or vascular impairment [1].Onycholysis and splinter hemorrhage are less in RT which could be of due to improvement metabolic changes and improving of ichting.Anemia are common in HD which lead to a change in the nail shape (koilonychia) and after renal transplantation anemia will improved and this could be related to decrease frequency of koilonychia. The pathogenesis of Muehrcke’s lines is still uncertain. Oedematous changes in the nail bed connective tissue [21] or alteration of the nail plate to nail bed attachment have been offered as possible explanations for the nail discoloration [22].Specific pathophysiologic mechanisms of clubbing remain unclear. It may be due to increased platelet derived growth factor and hepatocyte growth factor at the nail bed causing periosteal changes [23, 24].
5. Conclusions
- 1. Nail changes could be a marker of renal disease status.2. Absence of lunula is the most common nail changes in hemodialysis and renal transplant patients.3. Nail changes decreased in frequencies after renal transplantation like half and half nail, abscence of lunulaand and onycholysis apart from leukonychia, probably due to improvement of renal function.
References
| [1] | De Berker D A R, Baran R.. Disorders of Nails. In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rooks Textbook of Dermatology, 8th ed 2010. London Blackwell Publishing Company. 65, 1- 57. |
| [2] | Scher RK, Daniel CR . Nails Therapy, Diagnosis, surgery 2nd ed. Pheladelphia: WB Saunders, 1997. |
| [3] | Zaias N. Embryology of human nail. Arch. Dermatol 1963; 87: 37-24. |
| [4] | Lewis BL. Microscopic studies of fetal and mature nail and surrounding soft tissue. Arch. Dermatol 1954; 70: 732. |
| [5] | Lewin K. The normal finger nail. Br. J Dermatol 1965; 77: 421-4. |
| [6] | Graham A, Johnston Robin A, C Graham-Brown. Skin manifestation of internal organ disorders. In Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitz Patrics Dermatology in General Medicine 7th ed, Mc Graw-hill, 2008; 151:1458-1459. |
| [7] | Pieter Evenepoel and Dirk R .Kuypers. Dermatological Manifestation of Chronic Kidney Disease. In: Comprehensive Clinical Nephrology 3rd ed .MOSBY ELSEVIER, 2007, 78, 903. |
| [8] | ELSEVIER, 2007,68, 813-814. Mohsen EI Kossi, Meguid EI Nahas. Epidemiology and Pathophysiology of Chronic Kidney Disease: Natural History, Risk Factors, and Management. In Comprehensive Clinical Nephrology 3rd ed .MOSBY |
| [9] | Altmeyer P, Kachel HG, Junger M. Skin changes in long-term dialysis patients. Hautarzt 1982;33: 137–142. |
| [10] | Bencini PL, Montagnino G, Citterio A. Cutaneous abnormalities in uremic patients. Nephron 1985; 40: 316–321. |
| [11] | Kint A, Bussels L, Fernandes M, Ringoir S. Skin and nail disorders in relation to chronic renal failure. Acta Derm Venereol (Stockholm) 1974; 54: 137–140. |
| [12] | Pico MR, Lugo-Somolinos A, Sanchez JL, Burgos-Calderon R. Cutaneous alterations in patients with chronic renal failure. Int J Dermatol 1992;31: 860–863. |
| [13] | Yasemin Saray, MD, Deniz Sec¸kin, MD, Ayse Tu¨lin Gu¨lec¸, MD, Seval Akgu¨n, MD,b and Mehmet Haberal, MD, FACSc. Nail disorders in hemodialysis patients and renal transplant recipients: A case-control study. J Am Acad Dermatol (2004) ;50, 2, 197-202. |
| [14] | J.Parkash, S. Singh, G.K. Prashant, B. Kar, K. Tripathi, and P. B. Singh. Mucocutaneous Lesion in Transplant Recipient in a Tropical Country. Trasplantation Proceeding 2004, 36, 2162-2164. |
| [15] | Tercedor J, Hernandez BL, Rodenas JM. Nail diseases in hemodialysis patients: case–control study. Br J Dermatol 2001; 144: 445–446. |
| [16] | Iorizzo M, Pazzaglia M, Piraccini BM, Tullo S, Tosti A. Brittle nails. J Cosmet Dermatol, 2004; 3: 138–144. |
| [17] | Kint, A., Bussels, L., Fernandes, M. Skin and nail disorders (1974). 172,24-30. |
| [18] | Gilkes J., J.H., Eady R A.J., Rees LH. Plasma immunoreactive melanotrophic hormones in patients on maintenance haemodialysis. Br Med J (1975).1,656-658. |
| [19] | Stewart WK, Raffle EJ. Brown nail-bed arcs and chronic renal disease. Br Med J (1972); 1,784-786. |
| [20] | Albright SD, Wheeler CE. Leukonychia: total and partial leukonychia in a single family with review of the literature. Arch Dermatol 1964; 90: 392–9. Lewin, K. (1965) The finger nail in general disease. Br J Dermatol 37, 431-438. |
| [21] | Lewin, K. (1965) The finger nail in general disease. Br J Dermatol 37, 431-438. |
| [22] | Muehrcke’s lines. Archives of Internal Medicine 116,875-878. |
| [23] | Spicknall K, Zirwas MJ, English JC 3rd. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology and clinical relevants. J Am Acad Dermatol, 2005; 52: 1020–1028. |
| [24] | Martinez-Lavin M. Exploring the causes of the most ancient clinical sign of medicine: finger clubbing. Semin Arthritis Rheum, 2007; 36: 380–385. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML