-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2014; 3(2): 23-29
doi:10.5923/j.ajdv.20140302.01
Investigation of the Gene Expression Levels of 5 α-reductase, VEGF and IL-1α in HaCaT Cells after the Application of a Botanical Extract
Ahu Izgi, Murat Türkoğlu, Berrin Akyıldırım, Sabiha Çelen, Nuray Çelik
Biota Laboratories, Emek Mahallesi, Ordu Caddesi, Sancaktepe, 34785 Istanbul, Turkey
Correspondence to: Ahu Izgi, Biota Laboratories, Emek Mahallesi, Ordu Caddesi, Sancaktepe, 34785 Istanbul, Turkey.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background:Hair loss also known as alopecia is a common dermatological problem both for men and women all over the world. Deficiency in nutrition of the hair due to decrease in the micro-circulation in the scalp, or aging of the follicle cells due to lack of care are counted as significant causal reasons for hair loss. Treatment methods by using natural products such as plants and their derivatives are of great interest. In our study, HaCaT human keratinocyte cells were used as a model to demonstrate the effect of a botanical extract resulting from the specific plants of a unique Northeastern region of Turkey (Anzer) on gene expression of the cells.Material and Methods:Cell proliferation assay was performed by XTT reagent. After determination of non-cytotoxic concentration, cells were treated with plant mix and incubated for five days. RNA isolations were carried out from both non-treated and treated cell groups by using Tri-reagent. Gene expression of VEGF, IL-1a and 5-alpha reductase was determined by qPCR analysis.Results:The results demonstrated that Plant extract used in the current study revealed anti-inflammatory and anti-androgenic properties by downregulating IL-1α a and 5-α-reductase gene expressions, respectively. Furthermore, it led to upregulate VEGF gene expression which is a significant growth promoting factor for HaCaT cells.Conclusion: This plant mix seems to be a promosing candidate for preventing hair loss due to its anti-inflammatory, anti-androgenic and hair promoting properties.
Keywords: HaCaT cell line, Gene expression, VEGF, IL-1α, 5-α reductase
Cite this paper: Ahu Izgi, Murat Türkoğlu, Berrin Akyıldırım, Sabiha Çelen, Nuray Çelik, Investigation of the Gene Expression Levels of 5 α-reductase, VEGF and IL-1α in HaCaT Cells after the Application of a Botanical Extract, American Journal of Dermatology and Venereology, Vol. 3 No. 2, 2014, pp. 23-29. doi: 10.5923/j.ajdv.20140302.01.
Article Outline
1. Introduction
- Throughout history, plants have been used for nourishment, dyeing and medical purposes [1]. Turkey is one of the richest countries in the world in terms of plant diversity [2]. A number of etnobothanical studies are conducted in Turkey enabling the transfer of the knowledge on plant-based treatments for the future [3-6]. Of all the sectors, cosmetic industry benefits from botanical extracts more frequently. Botanical extracts that support integrity of the skin, hair, and nails are widely used in cosmetic formulations due to their anti-oxidant, anti-aging properties [7]. Bioactives in plant extracts offer a specifically exciting area for further research in terms of their molecular effects on cells and tissues. The skin is the largest organ covering the body surface and composed of keratinized stratified epidermis, collagen-rich dermal connective tissue which contains hair follicles, nails, sebaceous glands and sweat glands derived from the epidermis [8]. Hair originates from a follicular structure consisting three parts: an outer root sheat, an inner root sheat, the hair fiber itself [9]. Hair follicle is considered to be a significant model organ of interaction of epithelium and mesenchyma [10]. Dermal papilla cells are a population of mesenchymal cells in the skin and thought to be a reservoir of multipotent stem cells. Furhermore, they become specialized fibroblast cells during hair follicle differentiation [11]. Keratinocyte cells make up about 95% of cell mass of human epidermis and they are responsible for the production of cytokeratins and mucopolysaccharides. The hair fiber is composed of 10nm keratin filaments produced by keratinocyte cells in hair follicle [12]. The hair follicle undergoes a cycle of developmental period from a resting (telogen) phase to a growth (anagen) phase in which follicular keratinocytes proliferate rapidly, hair shaft thickens and elongates [13, 14]. On the other hand, dermal papilla cells themselves are thought to not divide. However, the number of cells in the dermal papilla increases during anagen, possibly as a result of replenishment from neighbouring cells of the dermal sheath [15]. The hair cycle is dependent on cyclic activation involving the proliferation, differentiation and apoptosis of a variety of hair-forming cells, all of which is regulated by a network of signaling molecules. This developmental cycle is complex and can be affected by many factors such as growth factors, hormones, immunomodulators [16, 17]. During catagen, the epithelial cells at the base of the follicle are known to undergo apoptosis; however dermal papilla cells are reported to remain intact and is pulled or migrates upwards, until it comes to rest next to the stem cells of the hair follicle bulge. This situation is reported to continue during telogen phase. In anagen phase, cells at the base of the follicle start to proliferate resulting in downward growth of the follicle and envelopment of the dermal papilla cells. Although it is stated that dermal papilla cells themselves do not divide, the number of cells in the dermal papilla increases during anagen, possibly as a result of replenishment from neighbouring cells of the dermal sheath [15].Dermal papilla cells not only retain the ability to form dermal papilla following in vitro culture, but they can also contribute to dermal sheath cells and non-follicle-associated fibroblasts during skin reconstitution and wound-healing [18]. However, after a few passages cultured dermal papilla cells lose their trichogenic properties (i.e. their ability to induce hair follicles) [19-23]. Therefore, not only dermal papilla cells but also keratinocyte cells are responsible for hair growth and differantiation.There are a number of significant growth factors leading hair growth such as vascular enthothelial growth factor, insülin like growth factors (VEGF, IGF). On the other hand, a number of factors such as tumor necrosis factor alpha, interleukin 1 alpha, interferon gamma (TNF-a, IL-1a, IFN-gamma) decrease the hair growth and/or lead to hair loss by increasing inflammation [24-30]. Among the hair promoting factors, VEGF, known to be responsible for blood vessel formation, has a particularly significant role in cell proliferation not only in endothelial cells but also in other certain types of cells. In addition, steroid 5 alpha reductase is a significant enzyme responsible for the conversion of testesterone into more potent 5-alpha dihydrotestesterone (5-alpha DHT) in specific tissues regulated by androgens [31]. Its expression was shown in epidermis, sweat glands, hair follicles, sebaceous glands [32].Anzer plateau is located on Zigana Mountains at altitudes up to 2500-3000m in Rize province of Turkey. The most striking feature of Anzer plateau is rich plant diversity. According to studies conducted in this region, 500 different types of plants have been found and approximately ninety of those plants are specific only to Anzer plateau. The honey obtained from various plants in Anzer plateau also known as “Anzer honey, medical honey” has been known for centuries to have a number of benefits on human health as a result of the unique vegetation in the region [33]. Therefore, it was hypothesized that the plants that were included in Anzer honey could also be a good candidate for skin and hair preparations. Many studies conducted sofar regarding the effects of plant extracts on keratinocyte cells showed different aspects of factors affecting on hair loss. However, anti-inflammatory effects and hair promoting properties of plant extracts on keratinocyte cells are still unclear. In the present study, a composion of different types of plant were used and applied to the cells. The effect of plant mix on the gene expression levels of 5-alpha reductase, VEGF and IL-1a were determined in human keratinocyte cell line (HaCaT) by using quantitative- real time polymerase chain reaction (q-RT-PCR).
2. Material and Methods
2.1. Materials
- The aerial parts of an annual or biennial herbaceous plants were collected from Anzer plateau in Rize province, İkizdere valley. Some plant species listed below in the Table 1. Woody, shrubs and bulbous plants were not included in this study since honey pollen analysis did not include them [34].
2.2. Preparation of Plant Extract
- Plants were collected on July of 2012 at İkizdere valley, Anzer region of Rize, Turkey. Collected plants were dried in open air by shade drying techniques [35]. Dried plants were fine-cut. 5 g of plant mixture collected from Anzer plateau was extracted with 20 mL distilled water for 15 minutes at 100°C. The extract was filtered through a filter paper into a sterile bottle. Subsequently, the solution was filtered through 0.45 µm membrane filter until it becomes clear.
2.3. Cell Lines and Culture
- The human keratinocyte cell line (HaCaT) was cultured in Dulbecco’s modified Eagle’s medium with high glucose containing 10% heat-inactivated fetal bovine serum (FBS) and 100U/ml penicillin and 100u/ml of streptomycin. The cells were maintained at 37℃ in a humidified atmosphere at 5% CO2 in Newbrunswick incubator. All supplements and media were purchased from Sigma Aldrich (Germany).
2.4. Cell Proliferation Assay with XTT Reagent
- HaCaT cells were seeded into 96 well plates (10000 cells/well) and were subjected to serial dilutions of plant extract to assess the cell proliferation.XTT and activator reagents were added to the plates after 72 hour incubation period was completed according to the manufacturer’s instructions. Then, cells were incubated at 37℃ for 4 hours in order that XTT reagent was reduced to formazan compound. The optical density of soluble formazan compound was measured at 495 nm with a BIO-RAD microplate reader (Japan).
2.5. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5.1. RNA Isolation and cDNA Synthesis
- Total RNA was extracted from cellstreated with plant extract and non-treated cells by using TRI-reagent according to manufacturers instructions (Sigma Aldrich, USA). The concentration and purity of isolated RNA samples were determined by measuring optical densities at 260 nm and 280 nm using Bio-spect nano (Japan). The intactness of isolated RNA and DNA contamination were checked by agarose gel electrophoresis.cDNA synthesis was performed with 5 mg total RNA, 20 pmol of gene specific primers of VEGF, IL-1a, 5 alpha reductase and GAPDH (Integrated DNA Technologies) and 40 units of M-MuLV reverse transcriptase according to the manufacturer’s instructions (Fermentas, USA).
2.5.2. Real Time Quantitative Polymerase Chain Reaction
- Real-time PCR (qPCR) reaction was carried out in Light Cycler 96 (Roche, USA). Amplification of products were detected via intercalation of the fluorescent dye SYBR green (Fast Start DNA Green Master. Roche, USA). Briefly, total volume of reaction mix was 20 ul containing 10 ul SYBR Green 2X master mix, 0.15 uM of reverse and forward primers, 2.8 uL of cDNA and appropriate amount of nuclease free water. All samples were run as triplicates in each run including a non-template control to check background signal. The PCR was run for 10 min at 95°C and followed by 45 cycles of 30s at 95°C, 30s at 57°C and 30s at 72°C; 30s at 95°C, 30 s at 58°C and 30s at 72°C; 20s at 95°C, 30s at 56°C and 30s at 72°C; 30s at 95°C, 30 s at 58°C and 35s at 72°C; GAPDH, VEGF, IL-1a, 5alpha reductase, respectively. After amplification, melting curve analysis was performed to verify specificity. Each run was carried out in triplicates including non-template controls. Delta delta Ct (2-DDCT) relative quantitation method was used for quantitation of qPCR products [36].
2.6. Statistical Analysis
- All data are representative of three experiments and expressed as mean ±standard error of the means (SEM). Statistical evaluation was performed by Student’st-test and one-way Anova using Graph Pad Prism 5 Software (USA) and post-hoc Tukey’s analyses were carried out to find groups those mean differences were significant. The results were significant at the 0.05 level.
3. Results
3.1. Cell Proliferation Analysis
- The cytotoxicity test is required for the determination of appropriate concentration required for the future work regarding the determination of gene expression levels, therefore the inhibitory concentration of plant extract on cell proliferation was determined. The anti-proliferative effect of plant extract was shown in Figure 1.
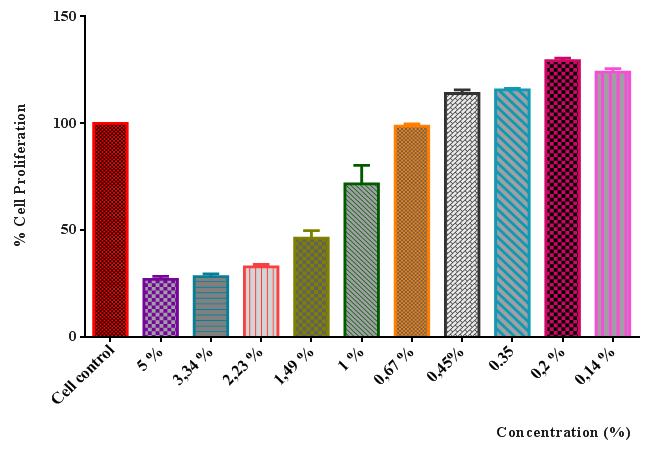 | Figure 1. The anti-proliferative effect of plant extract on HaCaT human keratinocyte cell line |
3.2. Expression Analysis of 5-alpha Reductase, IL-1a, VEGF
- According to RT-qPCR results, cells treated with plant extract showed 2.13 fold less mRNA expression of 5-alpha reductase gene compared to non-treated cells in the present study. (Figure 2). Plant extract seems to have an inhibitory effect on androgenic activity in HaCaT cells.
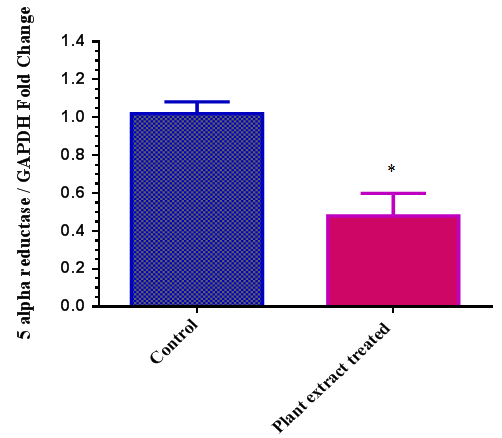 | Figure 2. Expression of 5 alpha reductase gene in HaCaT cells upon plant extract treatment. (* Results were signifcant with a p<0.05) |
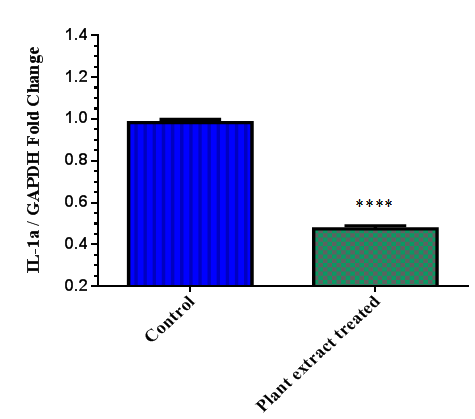 | Figure 3. Expression of IL-1a gene in HaCaT cellsupon plant extract treatment (**** Results with a p<0.0001) |
|
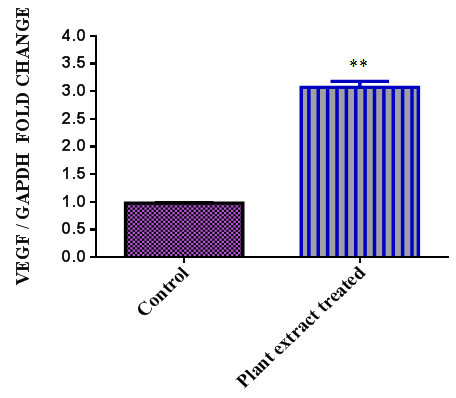 | Figure 4. Expression of VEGF gene in HaCaT cellsupon plant extract treatment (** Results with a p < 0.01) |
4. Discussion
- The Anzer Plateau has a huge plant diversity and the famous Anzer Honey is obtained from these plants. Anzer Honey is a well known medical honey in Turkey. Therefore, present study aimed to monitor the effects of the ingredients of the plants. Herbal extracts are added into cosmetic formulations because of their anti-oxidant, anti-inflammatory, anti-microbial properties. Up to date, many plants have been evaluated scientifically for their cosmetic potential and many herbal extracts were used in cosmetic products for skin whitening, hair care, hair loss, hair coloring [37-40]. Although a number of studies were conducted for some of the plants located at Anzer plateu but there was not a report indicating the effects of these plants for hair loss.Androgenetic alopecia (AGA) is characterized by shortening of the anagen phase and gradual thinning of hair density in the frontal and vertex scalp. Pathogenetic mechanisms in androgenetic alopecia are still unclear however, androgens are commonly accepted to inhibit growth activity of hair folliclewith early induction of the catagen phase [41, 42]. In human hair follicles, testosterone shows its effect either directly or after conversion by the enzyme 5 alpha reductase to the five times more potent 5-dihydrotestosterone (5-DHT).5-DHT is generally accepted to be responsible for the gradual miniaturization of genetically prone hairs with shortening of the anagen phase, thereby significantly reducing the anagen-to-telogen ratio [43-45]. Moreover, IL-1a plays important roles in a number of biological responses including inflammation and wound healing. In both cases, large amounts of IL-1 a reproduced by keratinocytes, fibroblasts and macrophages localized to the affected areas. Apart from those, several hair promoting factors were studiedsuch as VEGF, IGF-1 etc. and supposed to have significant effects on keratinocyte cells [45-52]. qRT-PCR has been used for the analysis of gene expression levels and it is a specific and sensitive method to analyse mRNA levels of genes. On the other hand, Western blotting method has been used for the protein analysis. In this study, we evaluated mRNA levels of three significant gene and found significant relations when the plant extract was applied to the cells. It may be useful to analyze protein levels of VEGF, IL-1a and 5 alpha reductase genes both in non treated and treated cells to see whether there would be a correlation between gene expression and protein expression levels. As a conclusion, using plant mix extract that contain plants from the famous Anzer plateau may have medicinal benefits againsthair loss.
References
| [1] | Davis PH (Ed.). Flora of Turkey and the East Aegean Islands. (1965-1985) Vol. 1-9, Edinburgh University Press, Edinburgh. |
| [2] | Ozgen U, Kaya Y, Coskun M. Ethnobotanical studies in the villages of the district of Ilıca (Province Erzurum), Turkey (2004). Econ. Bot. 58: 691-696. |
| [3] | Yesilada E, Sezik E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey (1999). IX. Folk medicine in north-west Anatolia. J. Ethnopharmacol. 64: 195-210. |
| [4] | Tuzlaci E, Tolon E. Turkish folk medicinal plants, part III: Sile (Istanbul) (2000). Fitoterapia. 71: 673-685. |
| [5] | Uzun E, Sariyar G, Adsersen A, Karakoc B, OtUk G, Oktayoglu E, Pirildar S. Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species (2004). J. Ethnopharmacol. 95: 287-296. |
| [6] | Ozgen U, Kaya Y, Coskun M. Ethnobotanical studies in the villages of the district of Ilıca (Province Erzurum), Turkey (2004).. Econ. Bot. 58: 691-696. |
| [7] | Stallings A.F, Lupo M.P. Practical Uses of Botanicals in Skin Care (2009). J Clin Aesthet Dermatol. Vol:2(1); 36–40. |
| [8] | Zhang J.T, Wang J.M. Progress in relevant growth factors promoting the growth of hair follicle (2012). American Journal of Animal and Veterinary Sciences 7 (2); 104-111. |
| [9] | Montagna W., Parakkal P.F. The structure and function of the skin (1974).3rd ed. Academic Press, New York and London; 172-258. |
| [10] | Yang C.C and Cotsarelis G. Review of hair follicle dermal cells (2010). J.Dermatol.Sci.,57;2-11. |
| [11] | Driskel R.R, Clavel C., Rendl M., Watt F.M (2011). Hair follicle dermal papilla cells at a glance.Journal of Cell Science 124, 1179-1182. |
| [12] | Lynch M.H, O’Guin W.M, Hardy C., Mak L., S.T.T. Acidic and basic hair/nail keratins (1986). The journal of Cell Biology.Vol.103;2593-2606. |
| [13] | Chase H.H. Growth of the hair (1954). Physiol. Rev.34:113-126. |
| [14] | Hardy M.H. The secret life of the hair follicle (1992).Trends Genet.8:55-60. |
| [15] | Tobin, D. J., Gunin, A., Magerl, M. and Paus, R. (2003). Plasticity and cytokinetic dynamics of the hair folliclemesenchyme during the hair growth cycle: implications forgrowth control and hair follicle transformations. J. Invest. Dermatol. Symp. Proc. 8, 80-86. |
| [16] | Yoo H.G, Chang I.Y, Pyo H.K et al. The additive effects of minoxidil and retinol on human hair growth in vitro (2007). Biological and pharmaceutical bulletin. Vol.30(1);21-26. |
| [17] | Baytop T. Therapy with medicinal plants in Turkey (1999). Nobel Medical Publish, Istanbul. |
| [18] | Biernaskie, J., Paris, M., Morozova, O., Fagan, B. M.,Marra, M., Pevny, L. and Miller, F. D. (2009). SKPsderive from hair follicle precursors and exhibit properties ofadult dermal stem cells. Cell Stem Cell 5, 610-623. |
| [19] | Ohyama, M., Zheng, Y., Paus, R. and Stenn, K. S. (2010).The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp. Dermatol. 19, 89-99. |
| [20] | Yang, C. C. and Cotsarelis, G. (2010). Review of hairfollicle dermal cells. J. Dermatol. Sci. 57, 2-11. |
| [21] | Horne, K. A., Jahoda, C. A. and Oliver, R. F. (1986).Whisker growth induced by implantation of culturedvibrissa dermal papilla cells in the adult rat. J. Embryol.Exp. Morphol. 97, 111-124. |
| [22] | Kishimoto, J., Burgeson, R. E. and Morgan, B. A. (2000). Wnt signaling maintains the hair-inducing activity of thedermal papilla. Genes Dev. 14, 1181-1185. |
| [23] | Rendl, M., Polak, L. and Fuchs, E. (2008). BMP signalingin dermal papilla cells is required for their hair follicleinductiveproperties. Genes Dev. 22, 543-557. |
| [24] | Konur A., Schulz U., Eissner G., Andreesen R., Holler E. (2005). Interferon-gamma is a main mediator of keratinocyte apoptosis and contributes to autocrine IFN-gamma and tumor necrosis factor-alpha production. Br. J. Dermatol. 152; 1134-1142. |
| [25] | Banno T.,Gazel A.,Blumenberg M.(2004). Effects of tumor necrosis factor-alpha in epidermal keratinocyes revealed using global transciptional profiling. J.Biol.Chem. 279; 326433-42. |
| [26] | Tavakkol A., Elder JT., Griffiths CE.,Cooper KD., Talwar H., Fisher GJ. (1992) Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1 and IGF-1 receptor mRNA and proteins in human skin. J Invest Dermatol. Vol: 99; 343-349. |
| [27] | Philpott M.P, Sanders D.A, Bowen J., Keaey T. Effects interleukins, colony-stimulating factor and tumor necrosis factor on human hair follicle growth in vitro; a possible role for interleukin-1 and tumour necrosis factor-a in alopecia areata (1996). British Journal of Dermatology 135; 942-948. |
| [28] | Kingston K., Mak L., Chan S.Y. Epidermal Growth Factor as a Biologic Switch in Hair Growth Cycle. The Journal of Biological Chemistry (2003). Vol, 278; 26120-26126. |
| [29] | Kimura-Ueki M. et al. Hair Cycle Resting Phase Is Regulated by Cyclic FGF18 Signaling (2012). Journal of Investigative Dermatology (2012) 132, 1338–1345. |
| [30] | Hoffmann R., Happle R. Current understanding of androgenetic alopecia. PartI: etiopathogenesis (2000). Eur J Dermatol.10;219-27. |
| [31] | Chen W., Zouboulis C.C., Fritsch M. et.al. Evidence of heterogeneity and quantitative differences of type 1 5 alpha reductase expression in cultured human skin cells (1998). The Society of Investigative Dermatology. Vol:110;1. |
| [32] | Luu-The V, Sugimoto Y, Puy L., Labrie Y., Solache IL., Singh M., Labrie F. Characterization, Expression Immunohistochemical Localization of 5-alpha reductase in human skin. J Invest. (1994) Dermatol.102:221-226. |
| [33] | Ulusoy, E., Kolaylı, S. Phenolic Composition And Antioxidant Properties of Anzer Bee Pollen. Journal of Food Biochemistry (2014) 38:73-82. |
| [34] | Ulusoy, E., 2010. Anzer Balı Ve Poleninin Yüksek Performanslı Sıvı Kromatografisi İle Fenolik Bileşiminin Belirlenmesi Ve Antioksidan Özellikleri. Karadeniz Teknik Üniversitesi Fen Bilimleri Enstitüsü Kimya Anabilim Dalı Doktora Tezi. |
| [35] | Terzioğlu, S., 1994. Of-İkizdere- Anzer Vadisi Florası. Karadeniz Teknik Üniversitesi, Fen Bilimleri Enstitüsü, Orman Mühendisliği Anabilim Dalı, Orman Mühendisliği Programı Yüksek Lisans Tezi. |
| [36] | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2-DDCT method. Methods 2001; 25:402–8. |
| [37] | Sorkun H., Doğan, C., 1995. Polen analysis of Rize - Anzer (Turkish) Honey. Apiacta 3:75-81. |
| [38] | Baytop T., 1999. Türkiye’de Bitkiler ile Tedavi, Geçmişte ve Bugün, Nobel Tıp Kitabevleri. ISBN: 975-420-021-1 |
| [39] | Kole P L., Jadhav H R., Thakurdesai P., Nagappa A N. 2005. Cosmetic potential of herbal extracts. Birla Institute of Science and Technology. Vol 4(4). |
| [40] | Ceres A, 1984. The healing power of herbal teas. Thursons Publishers, London. |
| [41] | Randall VA, Ebling FJ: Seasonal changes in human hair growth. Br J Dermatol 1991; 124: 146–151. |
| [42] | Ellis JA, Sinclair R, Harrap SB: Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med 2002; 19: 1–11. |
| [43] | Takayasu S, Adachi K: The conversion of testosterone to 17-hydroxy-5-androstan-3-one (dihydrotestosterone) by human hair follicles. J Clin Endocrinol Metab 1972; 34: 1098–1101. |
| [44] | Itami S, Kurata S, Sonoda T, Takayasu S: Characterization of 5 alpha-reductase in cultured human dermal papilla cells from beard and occipital scalp hair. J Invest Dermatol 1991; 96: 57–60. |
| [45] | Beato M. Gene regulation by steroid hormones. Cell 1989; 56: 335–344. |
| [46] | Messenger AG: The control of hair growth: an overview. J Invest Dermatol 1993; 101:S4–S9. |
| [47] | Sinclair RD, Dawber RP: Androgenetic alopecia in men and women. Clin Dermatol 2001; 19: 167–178. |
| [48] | Mizel, S. B. (1990) Zmrnunol. Today 11, 39W91. |
| [49] | Kupper, T. S. (1990) J. Invest. Dermatol. 94, 146S150S. |
| [50] | Kingston K., Mak L., Chan S.Y (2003) Epidermal Growth Factor as a Biologic Switch in Hair Growth Cycle. The Journal of BiologicalChemistry.Vol.278;26120-26126. |
| [51] | Tavakkol A., Elder JT., Griffiths CE.,Cooper KD., Talwar H., Fisher GJ. (1992) Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1 and IGF-1 receptor mRNA and proteins in human skin. J Invest Dermatol. 99:343-349. |
| [52] | Wang J.M, Zhang J.T (2012). Progress in Relevant Growth Factors Promoting the Growth of Hair Follicle. American Journal of Animal and Veterinary Sciences 7(2); 104-111. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML