-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2013; 2(3): 31-38
doi:10.5923/j.ajdv.20130203.04
Seroprevalence of Herpes Simplex Virus Type 2 (HSV 2) in Women with Bad Obstetric History
Zainab Khalil Mohamed Aljumaili1, Abdulghani Mohamed Alsamarai2, Wesam Suhail Najem3
1Department of Microbiology, Tikrit University College of Medicine, Kirkuk Health Authority, Tikrit, Iraq
2Departments of Medicine and Microbiology, Tikrit University College of Medicine, Asthma, Allergy Centre, Tikrit Teaching Hospital, Tikrit, Iraq
3Department of Dermatology, Tikrit University College of Medicine, Tikrit, Iraq
Correspondence to: Abdulghani Mohamed Alsamarai, Departments of Medicine and Microbiology, Tikrit University College of Medicine, Asthma, Allergy Centre, Tikrit Teaching Hospital, Tikrit, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
HSV 2 is common human pathogen that lead to lifelong latent infection and may be associate with transmission from mother to their fetus. The risk factors associated with HSV 2 seropositivity in pregnant women in Iraq are not known. The present study conducted to verify the prevalence of HSV 2 infections in women with bad obstetric history (BOH) in Kirkuk Governorate. HSV 2 seropositivity among women aged 17 to 47 years was investigated by determination of HSV 2 IgG and IgM in prospective a case control descriptive study. The overall HSV 2 seroprevalence was 24.2%, with a significant difference between women with BOH and women with normal pregnancy. HSV 2 IgM, as an indicator of current infection was demonstrated in 3.2% of the studied population, and was significantly higher in women with BOH compared to women with normal pregnancy. Both HSV 2 IgG and IgM were significantly varied with age groups, with trends of increasing with older ages. HSV 2 IgG was statistically significantly higher in working women as compared to housewife. Significant association was found between HSV 2 seroprevalence and family size and education levels.
Keywords: TORCH, HSV, BOH, IgM, IgG, Kirkuk, Iraq
Cite this paper: Zainab Khalil Mohamed Aljumaili, Abdulghani Mohamed Alsamarai, Wesam Suhail Najem, Seroprevalence of Herpes Simplex Virus Type 2 (HSV 2) in Women with Bad Obstetric History, American Journal of Dermatology and Venereology, Vol. 2 No. 3, 2013, pp. 31-38. doi: 10.5923/j.ajdv.20130203.04.
Article Outline
1. Introduction
- Perinatal infections account for 2% to 3% of all congenital anomalies. TORCH, which includes Toxoplasmosis, Other(syphilis, varicella-zoster, parvovirus B19, Human Immunodeficiency Virus and Hepatitis B virus), Rubella, Cytomegalovirus, and Herpes infections, are of the most common infections associated with unfavorable outcome of pregnancy[1].Herpes genitalis is one of the most common sexually transmitted diseases[2]. Herpes simplex virus type 2 (HSV-2) is the major cause of genital herpes[3]. The incidence of herpes simplex virus (HSV) infection has been increasing steadily in recent decades, and concerns about perinatal HSV infection are growing among women of reproductive age because of the risk of transmission of the virus to their babies during pregnancy, with potentially devastating consequences to the fetus[4]. Little is known about the seroprevalence of HSV 2 in Iraqi women with bad obstetric history[5-7]. These three studies reported a wide range of HSV 2 seropositivity (range from 8.1% to 73.9 for IgM), while other study for Iraq, reported seroprevalence of 28.9% HSV 2 IgM in pregnant women[8]. In addition, the study population of these 3 studies ranged from 100 to 162 subjects, which is lower that sample size required for HSV 2 seroprevalence study. Furthermore, the studies performed in Arab countries reported a range of 0.5%[9] to 7.6%[10] for HSV2 IgM and 6.5%[9] to 27.1% [11] for HSV2 IgG in pregnant women.The literature review[12] highlights a gap in existing knowledge on the epidemiology and impact of maternal infection, especially on the aetiology of infectious agents that lead to puerperal sepsis and subsequent mortality. Increased surveillance and diagnostic capabilities in healthcare facilities and in the community is needed to identify the aetiological agents responsible for puerperal sepsis and maternal mortality. The prevalence of maternal infection reported by the studies identified in literature regarding HSV 2 may be an underestimate of actual rates of infection as not all pregnant women in Iraq may have access to or choose to access formalized antenatal care. This could be due to financial constraints, difficulties in accessing these facilities and personal or cultural beliefs. In addition, antenatal care services may not have the capacity to routinely screen for maternal infections, especially those that are asymptomatic and those that require serological tests such as PCR and ELISA to diagnose, due to limited resources or expertise. These infrastructural problems are essential contributors to the persistence of high maternal morbidity and mortality in developing countries and need to be overcome in order to accurately characterize the burden of maternal infections in these countries, including Iraq[12].This literature review highlights the high bacterial and viral maternal infection rates in the developing world. Urgent, concerted action is required to reduce the burden of these infections. In addition to raising awareness about the severity of the problem of maternal infections in Iraq , data from seroepidemiological research will be beneficial in guiding public health policy, research interests and donor funding towards achieving improvement in health care delivery[12]The aim of this study was to identify seroprevalence of HSV 2 IgG and IgM in women with bad obstetric history compared to those with normal pregnancy and the association of these markers with socio-demographic variables of Iraqi population in Kirkuk Governorate.
2. Patients and Methods
2.1. Settings
- Kirkuk General Hospital, Primary Health Care Centers in Kirkuk Governorate.
2.2. Study Design
- The study design is a Descriptive Case Control Study.
2.3. Study Area
- This descriptive case-control study was conducted at the antenatal clinic of Kirkuk General Hospital and Primary Health Care Centre in Tessean. Women (Pregnant or Non pregnant) with bad obstetric history was recruited from those attending outpatient Gynaecology Clinic Kirkuk General Hospital or the outpatient Clinic at Tessean PHC.
2.4. Study Population
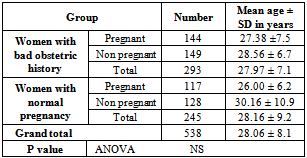
- The study population is women with childbearing age. Study population was recruited from Primary Health care Centers located in urban and rural areas in Kirkuk Governorates. In addition, one of the study population group was recruited from pregnant women who were in labor to select the group of pregnant with risky outcomes. Group 1: Pregnant women with age range of 15-48 years, and with normal pregnancy.Group 2: Non pregnant women with age range of 15-48 years, and with normal pregnancy.Group 3: Pregnant women with Risk factor (BOH) depending on their previous pregnancy and /or delivery outcome which include pregnancy loss, intrauterine deaths, preterm deliveries and intrauterine growth retardation. Their age range from 15-48 years.Group 4: Non- pregnant women with Risk factor depending on their previous pregnancy and /or delivery outcome which include pregnancy loss, intrauterine deaths, preterm deliveries and intrauterine growth retardation. Their age range from 15-48 years.The demographic information of these groups are shown in Table 1. The target number recruited for each group was 150 women. However, the total number of women included in the study was 538, of them 293 (54.5%) were with BOH, and 245 (45.5%) were with normal pregnancy history. In the BOH group, 144 (49.1%) women were pregnant, while in the normal pregnancy group, 117 (47.7%) were pregnant.
|
2.6. Collection of Data
- The designated investigators visited the outpatient department daily, selected the study subjects, and screened them using a predesigned pretested schedule considering the inclusion and exclusion criteria till the study subjects recruitment could be identified. The next available age-matched multiparous antenatal woman without BOH was included in the control group subjects.Clinical examination and laboratory investigations were carried out for the study subjects to exclude other causes of foetal wastage, such as hypertension, diabetes mellitus, syphilis, Rh (rhesus) incompatibility, physical causes of abortion, and consanguinity. Subjects with known causes of foetal wastage were excluded from the study. All of them were interviewed to ascertain age, medical and obstetric information.
2.7. Sample Collection
- For serological analysis, 5-10 mL of venous blood was collected in a sterile container with strict aseptic precautions from each study subject. The serum was separated and stored in numbered aliquots at -20℃ till assayed. All the serum samples collected from the study and control groups were tested for HSV 2 IgM and IgG antibodies by commercially- available (ELISA) kits. The results read by a Microwell reader and compared in a parallel manner with controls; optical density read at 450 nm on an ELISA reader.
2.8. Ethical Approval
- The ethical committee of the concerned institute approved the research protocol. The purpose and procedures of the study are to be explained to all the study subjects, and informed consent is to be obtained from them. The study design was approved by the ethical committee of TUCOM that was now registered in USA[U.S. Department of Health and Human Services (HHS) & Registration of an Institutional Review Board (IRB)]. IORG #: IORG0006885.Institution: Tikrit University College of Medicine [TUCOM] OMB No. 0990-0279
2.9. Methods
- ELISA was used for determination of IgM and IgG for HSV-2 and the test was performed according to manufacturer instructions. The kit purchased from BioCheck, Inc, 323 Vintage Park Dr, Foster City, CA 94404.
2.10. Analysis of Data
- Collected data were compiled in Microsoft Excel spreadsheet. The proportion and the mean value were computed in appropriate situations. To find out any association between categorical data, Chi square test was employed using the SPSS (Version 16). If the sample size in BOH group not reach the targeted number Power Analysis were performed to determine the accuracy of findings.The study finding data were presented as frequency ± SD and 95% Confidence Interval. Bivariate Regression Line Analysis to calculate Odd Ratio for determination of association between two variables. The determinants for HSV 2 infection is determined by calculation of Odd Ratio using Logistic Regression Line Analysis. Confounding factors such as age, socio-economic status, e.t.c are standardized when serological determinants were calculated.
3. Results
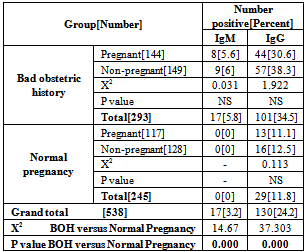
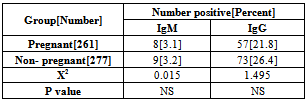
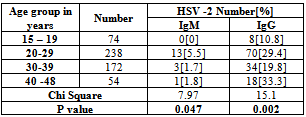
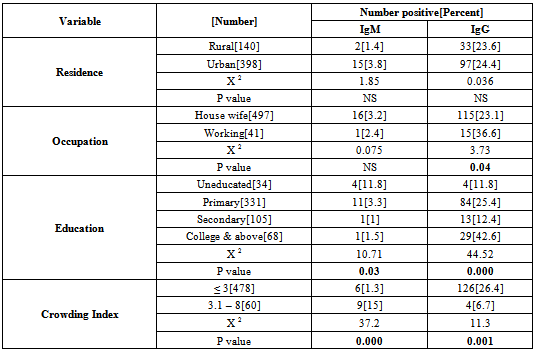
- A total of 600 women were recruited to study, as our target number is 150 women in each group. However, 62 women were defaulted from the study, 7 in women with BOH, and 55 in group of women with normal pregnancy. Thus the study population included in the statistical analysis was as shown in Table 1. There was no significant differences in mean of age between the study groups.The overall HSV 2 seroprevalence in our study population was 24.2%, with a significant (X2 =37.303, P=0.000) difference between women with BOH (34.5%) and women with normal pregnancy (11.8%). However, there was no significant difference between pregnant and non pregnant women in both BOH and normal pregnancy outcome groups. Table 2. HSV 2 IgM, as an indicator of current infection was demonstrated in 3.2% of the studied population, and was significantly (X2=14.67, P=0.000) higher in women with BOH (5.8%) compared to women with normal pregnancy (0%). There was no significant difference in HSV 2 IgG and IgM seroprevalence between pregnant (IgG =21.8%, IgM=3.1%) and non pregnant (IgG= 26.4%, IgM=3.2) women. Table 3. Both HSV 2 IgG and IgM were significantly varied with age groups, with trends of increasing with older ages (X2=15.1,P=0.002 for IgG; X2=7.97, P=0.04 for IgM). HSV 2 IgG seroprevalence was lower in women with age of less than 20 years (10.8%), subsequently, increased in women with age of 20-29 years (29.4%), with a higher seroprevalence in women with age of 40-48 years. HSV 2 IgM was not detected in women with age of less than 20 years, however, the higher seroprevalence rate (5.5%) was in women with age of 20 – 29 years. Table 4. OR calculation not confirm the association between HSV 2 IgG and IgM seropositivity and women age of less than 30 years. Table 5.
|
|
|
|
|
|
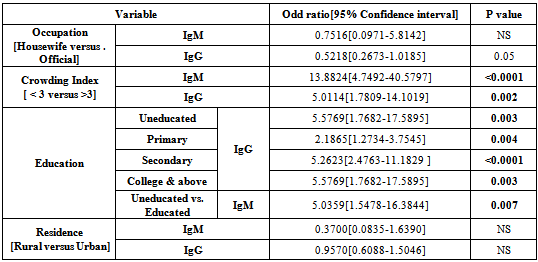
- HSV 2 IgG was statistically significantly (P=0.04) higher in working women (36.6%) as compared to housewife (23.1%), however, OR not confirmed an association with mother occupation (OR=0.5218, P=0.05). Tables 6 & 7. Mother education do influence significantly HSV 2 IgG (X2=44.52, P=0.000) and HSV 2 IgM (X2=10.71, P=0.03) seropositivity and OR confirmed a significant association between education levels and IgG (OR=2.18 – 5.57, P=0.004-0.0001) and IgM seropositivity (OR= 5.03, P=0.007). Tables 6 & 7.Family size significantly influence HSV 2 IgG (X2=11.3, P=0.001) and IgM (X2=37.2,P= 0.000) seroprevalence and such association was confirmed by OR (IgM, OR=13.88, P<0.0001; IgG, OR=5.01,P=0.002). Tables 6 and 7.
4. Discussion
- Infection with herpes simplex is one of the most common sexually transmitted infections. Because the infection is common in women of reproductive age it can be contracted and transmitted to the fetus during pregnancy and the newborn. Herpes simplex virus is an important cause of neonatal infection, which can lead to death or long-term disabilities. Rarely in the uterus, it occurs frequently during the transmission delivery[13]. The greatest risk of transmission to the fetus and the newborn occurs in case of an initial maternal infection contracted in the second half of pregnancy. The risk of transmission of maternal-fetal-neonatal herpes simplex can be decreased by performing a treatment with antiviral drugs or resorting to a caesarean section in some specific cases[13].Some trends may be drawn from a review of type-specific HSV prevalence in different geographic areas and subpopulations[14]. First, HSV-2 prevalence is highly variable and depends on many factors, including country and region of residence, population subgroup, sex, and age. Second, HSV-2 prevalence is, in general, higher among higher risk sexual behavior groups. Third, HSV-2 prevalence is generally higher in women than men. Fourth, HSV-2 prevalence is strongly associated with age, increasing from negligible levels in children younger than 12 years to as high as 80% among higher risk populations. Fifth, in a given population and age group, HSV- 1 prevalence is almost always greater than HSV-2 prevalence. Striking variations in HSV-2 prevalence were noted in different geographic regions. HSV-2 prevalence is highest in areas of Africa and parts of the Americas. In western and southern Europe, HSV-2 prevalence is usually lower than in northern Europe and North America. In Asia, HSV-2 prevalence appears lower than in other geographic areas. Direct comparisons in overall prevalence from independent studies should be made with caution given substantial differences in the populations surveyed, age distributions, and HSV serologic test methods.Comparisons of age-specific HSV-2 prevalence among similar populations in different regions may be useful and further data acquired by using the same serologic methods would be desirable. HSV-2 prevalence was consistently higher in higher risk populations compared with those considered at a lower risk. In some countries such as the USA, HSV-2 prevalence was strongly dependent on race; black Americans had a much higher prevalence of infection than whites and Mexican Americans of all ages. These results suggest that within populations, the risk of acquiring HSV-2 infection is highly variable[14]In the present study, an overall HSV IgG seroprevalence of 24.2% was found among women, and it was significantly (X2= 37.303, P=0.000) higher in women with BOH (34.5%) as compared to women with normal pregnancy (11.8%). However, there was no significant difference in seroprevalence between pregnant and non pregnant women, but was higher in non pregnant than pregnant women. This study HSV 2 seroprevalence (11.82%) in pregnant women was about similar to that reported for China,(10.8%)[15], Indonesia (9.9%)[16], Bangladesh (9.91%)[17], and UK (10.4%)[18]. In contrast to our study, a much higher HSV 2 seroprevalence has been reported from Zimbabwe (51.1%)[19], Germany (82%)[20], Turkey (63.1%)[3], and Iran (43.75%)[21]. In addition, our finding was lower than that that reported for Tanzania (20.7%)[22], Australia (30%) [23], USA (22%)[24], Switzerland (21.2%)[25], Canada (17.3%)[26], Senegal (22%)[27], Belgium (18.2%)[28] , China (23.5%)[29], and Korea (17%)[30]. However, the present study seroprevalence was higher to that reported for Turkey (4.4%-5%)[31,32], Kashmir (7.5%)[33], India (8.7%)[34], Croatia (6.8%)[35], Italy (7.6-8.4%)[13].In women with BOH, our HSV2 IgG seroprevalence (34.5%) was about similar to that reported for India(33.58%) [36] and Nepal (33.3%)[37]. However, it was higher to that reported for India (16.8-18.6%)[38,39]. Furthermore, the seroprevalence was much lower to that reported for Waset (60.6%), Iraq[6] in women with spontaneous abortion. In pregnant women, this study HSV IgG seroprevalence was lower to that reported for Saudi Arabia (27.1%)[11], Qatar (26.3%)[10], Babylon, Iraq (22.2%)[8] and Syria (52%)[40]. However, it was higher to that reported other two studies in Saudi Arabia (6.5-6.8%) [9,41]. The HSV 2 IgG seroepidemiology varies between different countries, and between groups of individuals depending on the demographic and clinical characteristics of the population. For example, studies performed in Iraq (11.1-60.6%), Saudi Arabia (6.5-27.1%), and Turkey (4.4%-63.1%), demonstrated a wide range of seroprevalence. These variations may be attributed to various sexual behavior, number of previous pregnancies, duration of sexual activity, residence, education, occupation and socioeconomic status[33,34,41-44] Seropositivity in the present study was found to be highly associated with history of previous abortion[X2=37.303, P=0.000), a finding agreed to that reported by others[43,45]. In our study, the HSV 2 IgG seroprevalence rose steadily with age (10.9% among women aged 15-19 to 29.4% among women aged 20-29 years. There was a significant variation (X2=15.1, P=0.002) in HSV 2 IgG seroprevalence between age groups. These findings are comparable to studies reported for other geographical areas[3,23,28,33,41-52]. Residence not seems to influence HSV 2 seroprevalence as this study demonstrated a non significant differences between rural (23.6%) and urban (24.4%) areas. OR not confirmed an association between residence and HSV 2 seroprevalence. This findings agreed to that reported by Rathore et al[33] , however, Chawla et al[48] suggested an association between residence (urban middle class) and HSV 2 seroprevalence.HSV 2 IgG seroprevalence was significantly (X2=3.73, P=0.04) higher in working women (36.6%) as compared to housewife (23.1%) women, however, OR not confirm such association. These findings agreed to that reported by others[32,33,34,52], while one study from Saudi Arabia[41] reported an association, but when the data grouped as we do, no such significant association was achieved.HSV 2 IgG seroprevalence was significantly (X2= 44.52, P=0.000) varies according to women education levels in our study and this association was confirmed by OR using bivariate analysis (OR= 2.18 – 5.57, P=0.004-<0.0001). The seroprevalence was steady increased with education, same to that reported by Chawla et al[48] and Xu et al[47], while other studies show high seroprevalence in less educated women[46,41,42]. However, Biswas et al[34] reported higher incidence in women with secondary school education. Page et al[53], showed the highest prevalence of HSV 2 in women with the lowest education level residing in the highest socioeconomic status area. Rathore et al[33] and Agabi et al[52] not found a significant association between HSV seroprevalence and education levels. In this study, HSV IgG seroprevalence was significantly higher (X2=11.3, P=0.001) in small size families (crowding index) as compared to large size families and this association confirmed by OR calculation (OR=5.0114, P=0.002). HSV infection is increased with the increase in sexual activity and thus small size families may provide comfortable environment that encourage sex performance. In addition, young women receiving family planning services are at risk for herpes simplex virus type 2 (HSV-2) infection[54]. The literature indicated a paradox in association between HSV 2 seropositivity and lower income and this could be due to differences in risk behavior among the different income groups. It was seen in a study performed for India[34], that majority of Muslims subjects (84.9%;90/106) were from low income group. It was also observed that Muslims subjects had the lowest HSV 2 seroprevalence (3.8%) compared to Hindu (5.8%) and Christians (12.6%), which may explain that disparity. The suggested risk factors that lead to high HSV 2 seropositivity in developed and some undeveloped countries are not applicable in our society due to religious and social reasons. Thus other risk factors are to be speculated in our society, one of these is the male circumcision, as it lowers the prevalence of HSV 2[55]. Recently reported study[33], higher HSV 2 seroprevalence was found among Christians versus Muslims and this differences in prevalence with religions, may be due to practice of male circumcision at infancy or early childhood by the spouses of the pregnant women among Muslims.Low socioeconomic status observed in some studies to be associated with HSV 2 seroprevalence[33,34,44,48,53]. However, Germany as a country with high socioeconomic status, the HSV 2 seroprevalence was 82% in pregnant women, while the corresponding value in Arab countries ranged from 6.5% to 27.1%. Thus Islamic legislation concerning faithful family relations and personal hygiene are an important factors that reduce HSV 2 infection.HSV 2 IgM seroprevalence was 3.2% indicating that current infection of 3.2% in our study population, and it was significantly higher (X2=14.67, P=0.000) in women with BOH (5.8%) as compared to women with normal pregnancy (0%), and about the same in pregnant and non pregnant women. Our HSV IgM (3.1%) seroprevalence in pregnant women was higher than that reported for Turkey (0%)[31], Saudi Arabia (0.5%)[9], Croatia (1.2%)[35], and Bangladesh (1.8%)[17]. Higher seroprevalence was reported for Babylon, Iraq (28.9%)[8], Turkey (13.8%)[3], and Qatar (7.6%)[10].In women with BOH, HSV IgM seroprevalence (5.8%) was lower than that reported for India[38,56] , Baghdad, Iraq[5], Waset, Iraq[6], Mosul, Iraq[7] and higher to that reported in two studies in India[36,57]. Thus current infection with HSV 2 was lower to that reported in other Iraqi Governorates. The present study shows a significant variation in current HSV 2 infection between age groups (X2=7.97, P=0.047), the highest incidence in women with age of 20-29 years (5.5%) old, while the lowest rate in women of <19 years old. Using Bivariate analysis , OR not confirmed an association between HSV 2 current infection and age of <30 or > 30 years old, residence, and occupation. However, current infection of HSV 2 was significantly higher (X2=37.2, P=0.000) in large size families (> 3 crowding index) than small size families. OR confirmed the association between current HSV infection and family size (OR=5.0114, P=0.002).
5. Conclusions
- The present study indicated a significant association between HSV 2 IgG and IgM and bad obstetric history. The overall HSV 2 seroprevalence in our study population was 24.2%, with a significant difference between women with BOH and women with normal pregnancy. Significant association was found between HSV 2 seroprevalence and family size and education levels. However, HSV 2 seroprevalence was different due to residence, occupation and age of < 30 years, but OR not demonstrate significant association.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML