-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2013; 2(3): 27-30
doi:10.5923/j.ajdv.20130203.03
Primary Cutaneous Follicular Lymphoma Associated with Helicobacter pylori Infection
A. H. Bashir1, 2, S M Yousif3, Lamyaa A M EL Hassan4, W M Elamin5, Ameera Adam6, M. E. Ibrahim6, K. O. Alfarouk6, 2, A. K. Muddathir7, A M EL Hassan6
1Khartoum collage of Medical Science, Khartoum, Sudan
2H. Alfarouk Cancer Center, Khartoum, Sudan
3Aliaa Medical Centre, Khartoum 3, Khartoum, Sudan
4School of Medicine, Ahfad University for Women, Um Durman, Sudan
5Faculty of Medical Laboratory Sciences, Alzaeim Alazhari University, Khartoum, Sudan
6Institute of Endemic Diseases, University of Khartoum
7Faculty of Pharmacy, University of Khartoum
Correspondence to: A. H. Bashir, Khartoum collage of Medical Science, Khartoum, Sudan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A 66 year old male with a long standing uncontrolled gastric H pylori infection and Crohn’s disease presented with nodular lesions in the back. These were removed surgically. Pathologically the lesion consisted of lymphocytes, giant cells with vacuolated cytoplasm and histiocytes. By immunohistochemistry there were stem cells, B cells and CD1a Langerhans cells. The diagnosis of Langerhans histiocytosis was made. The giant cells were positive for both CD 20 B cell marker and the macrophage marker CD 68 indicating that they were derived from B cells. They were strongly positive for H pylori antigen. A year later the patient reported with non-itching nodular lesions in the right flank. There was no lymphadenopathy or Splenomegaly. A biopsy of the lesion showed a follicular center B cell lymphoma. The tumor cells were positive for H pylori antigen. He was treated for H pylori infection. He completely recovered and was in good health a year later.
Keywords: Langerhans Histiocytosis, H. pylori infection, Cutaneous Follicular B cell lymphoma
Cite this paper: A. H. Bashir, S M Yousif, Lamyaa A M EL Hassan, W M Elamin, Ameera Adam, M. E. Ibrahim, K. O. Alfarouk, A. K. Muddathir, A M EL Hassan, Primary Cutaneous Follicular Lymphoma Associated with Helicobacter pylori Infection, American Journal of Dermatology and Venereology, Vol. 2 No. 3, 2013, pp. 27-30. doi: 10.5923/j.ajdv.20130203.03.
1. Introduction
- Helicobacter pylori infectionis a major cause of chronic gastritis, peptic ulcer disease, gastric cancer and gastric mucosa-associated lymphoid tissue (MALT) lymphoma[1]. MALT lymphoma arises from the marginal zone of the lymphoid follicle. Pathologically, they consist of centrocyte-like cells, and lymphoepithelial lesions[2, 3]. In addition to gastric and intestinal involvement, MALT lymphoma has been described in other tissues that include the skin,lung, thyroid gland, conjunctiva, orbit and salivary glands[4-6]. Herein we describe a patient with a long history of H pylori infection who developed a localizedH. pylori associated cutaneous Langerhans histiocytosis that was excised surgically. A year later he developed a follicular B cell lymphoma in a different part of the skin. It was associated with H pylori infection and resolved completely after surgical removal and anti H. pylori drug therapy. As far as we are aware this is the firstfollicular B cell lymphoma of the skin associated with cutaneous H. pylori infection. The pathogenesis of the lesion is discussed
2. Case Presentation
- A 66 year old male with a long standing uncontrolled gastric H pylori infection and Crohn’s disease presented with nodular lesions in the back. These were removed surgically. The excised lesion was examined histologically in H&E stained sections and by immunohistochemistry. All reagents for the latter were from Dako, Denmark. Histopathology showed small lymphocytes andhistiocytes; some of the latter were large giant cells with foamy cytoplasm (Fig 1). Based on the H&E stained sections the initial diagnosis was xanthogranuloma. The cells with dendrites were positive for CD1a (Fig 2). Based on this finding the diagnosis of Langerhans histiocytosis was made.The giant cells and some smaller cells were positive for the histiocytes marker CD 68 (Fig 3). The giant cells were also positive for the B cell marker CD 20 (Fig 4). Thus these cells are immature cells that may differentiate into B cells or macrophages depending on the stimulus. Some were strongly positive for H pylori antigen (Fig 5)(Dako Denmark).Some cells were positive for S-100 protein. Scattered CD 34-positive stem cells were identified (Fig 6). There were also CD 3 positive T cells.
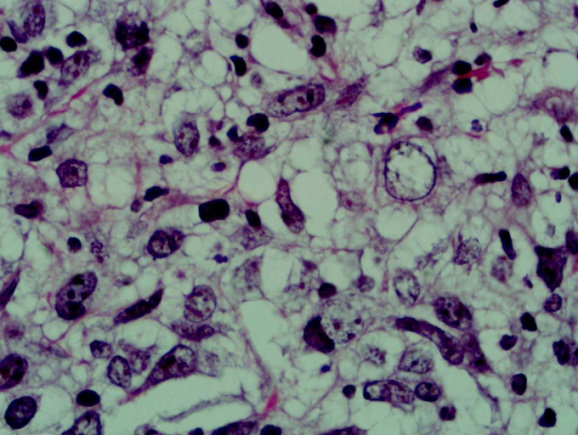 | Figure 1. shows lymphocytes, histiocytes and vacuolated giant cells (H&Ex40) |
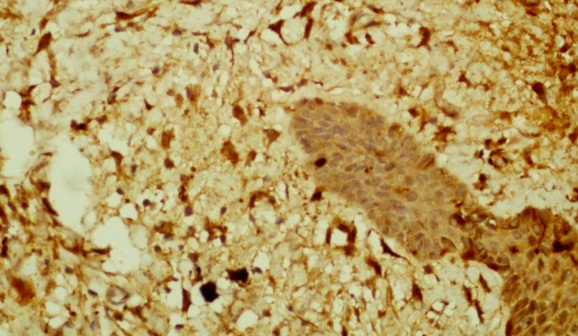 | Figure 2. shows CD1a positive dendritic cells in the dermis and epidermis (Immunohistochemistry x 40) |
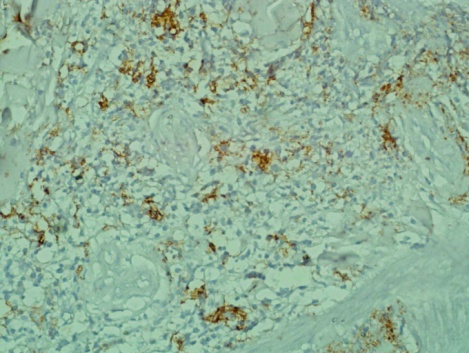 | Figure 3. shows the giant cells are positive for the macrophage marker CD 68. (Immunohistochemistry x10) |
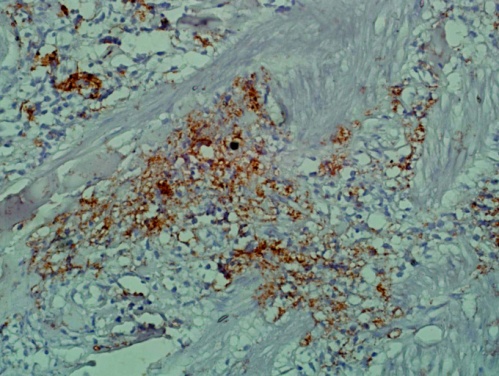 | Figure 4. shows the giant cells and the small lymphocytes are positive for the B cell marker CD 20. (Immunohistochemistry x 10) |
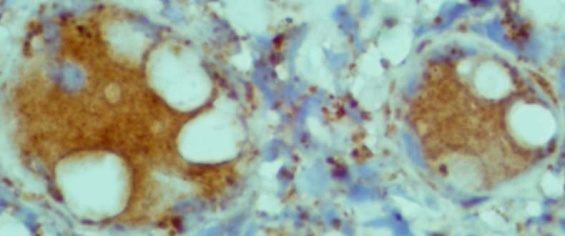 | Figure 5. shows the giant cells and macrophages are strongly positive for H pylori antigen. (Immunohistochemistry x100) |
 | Figure 6. shows CD34 positive stem cells. Small vessels are also positive and act as internal control> (Immunohistochemistry x 40) |
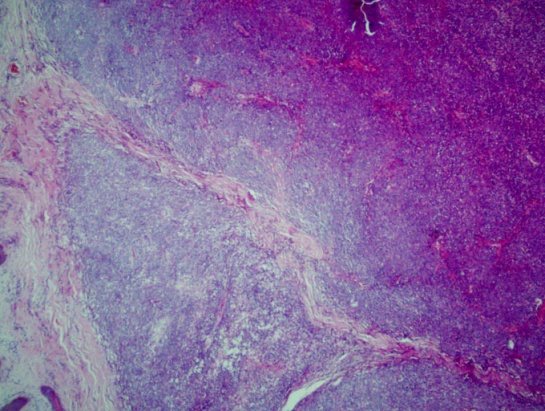 | Figure 7. shows nodules of lymphoid cells (H&E x10) |
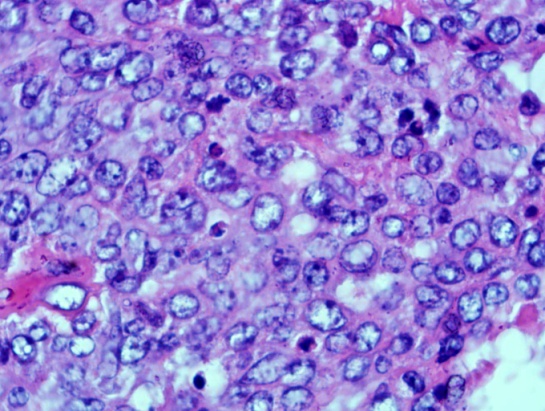 | Figure 8. shows that the tumour cells are a mixture of centrocytes and centroblasts (H&E x40) |
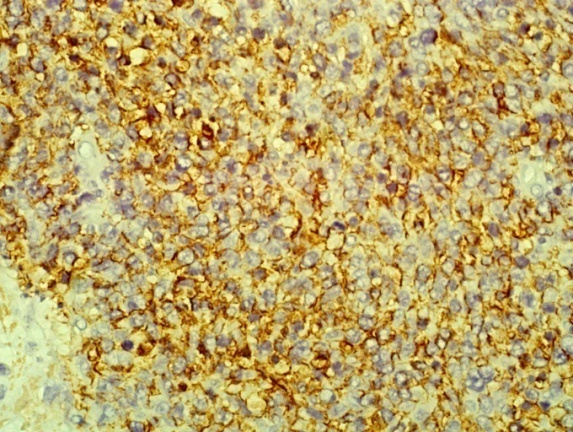 | Figure 9. shows the tumour cells are positive for the B cell marker CD 20. (Immunohistochemistry x 40) |
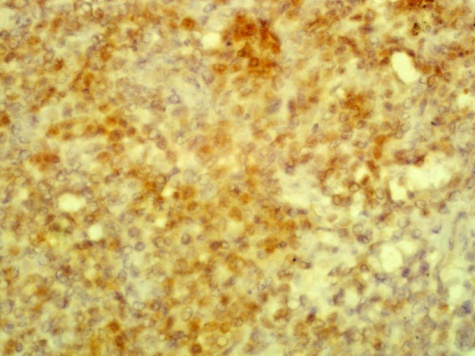 | Figure 10. A focus shows BCL2 positivetumour cells. (Immunohistochemistry x 40) |
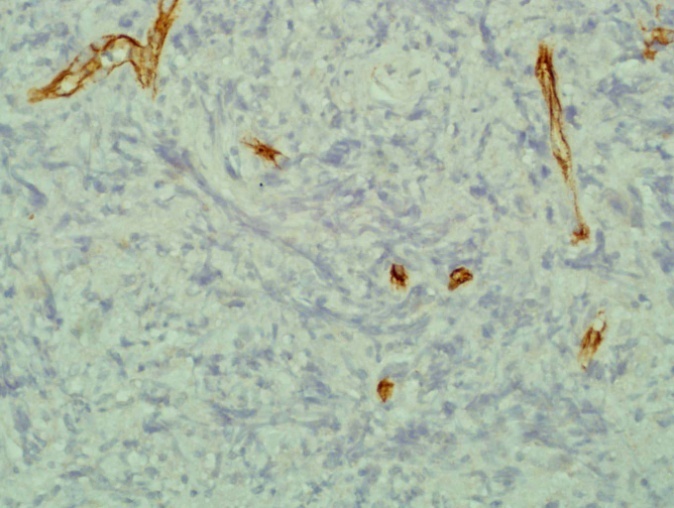
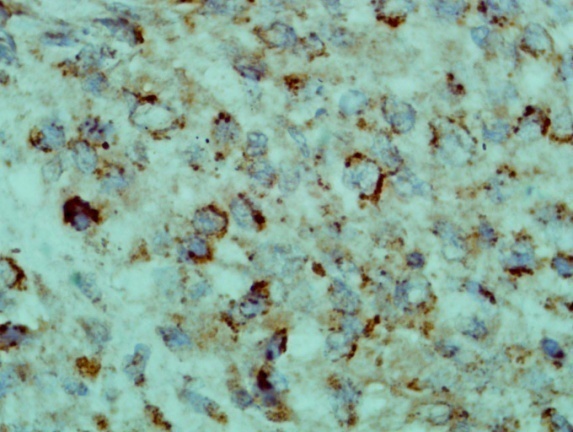 | Figure 11. shows the tumour cells are expressing H pylori antigen on the cell membrane (Immunohistochemistry x 40) |
3. Discussion
- The patient presented here is unique and demonstrates a spectrum of diseases associated with H pylori infection. These included poorly controlled gastric H pylori infection, long standingCrohn’s disease, cutaneous Langerhans histiocytosis and cutaneous follicular center cell lymphoma. We previously reported the association of H pylori infection with Langerhans histiocytosis in a 25 years old female with cutaneous lesions of 10 years duration[7]. Helicobacter pylori was reported in the intestinal mucosa of a patient affected by an ulcerative colitis-like phenotype of Crohn's disease (CD)[8]. It is doubtful if H pylori infection is a cause of Crohn’s disease. Some patients develop Crohn’s disease after eradication of the organism[9]. For some, this suggested that H pylori may protect against Crohn’s disease [10]. Our patient had irregular anti H pylori therapy with improvement and recurrences. When we saw him first we diagnosed his skin lesions as xanthogranuloma. After the demonstration of CD 1a positive cells by immunohistochemistry we changed our initial diagnosis to Langerhanshistiocytosis.A complex overlap exists between xanthogranuloma and Langerhans cell histiocytosis, with lesions showing clinical and/or pathological features of both disorders[11].The types of cells in the initial biopsy were a mixture of interrelated cells. We identified stem cells that were most probably the origin of the various cells. The cells showing the B cell marker were small lymphocytes and surprisingly the large foamy histiocytes were positive for CD 20. It is known that some histiocytes are derived from B cells. It was shown that enforced expression of C/EBPα and C/EBPβ in differentiated B cells leads to their rapid and efficient reprogramming into macrophages[12]. C/EBPs induce these changes by inhibiting the B cell commitment transcription factor Pax5, leading to the down-regulation of its target CD19, and synergizing with endogenous PU.1, an ETS family factor, leading to the up-regulation of its target Mac-1 and other myeloid markers[12]. We suggest that some of the giant cells co-expressing CD 68 and CD 20 had their CD marker down regulated but they expressed the CD 68 macrophage marker.The Langerhans cells are derived from the epidermis. There were CD1a negative but S100 positive dendritic cells in the dermal infiltrate.Human dendritic cells (DCs) represent a rare and heterogeneous population of cells that originate from CD34+ hematopoietic stem cells. Myeloid DCs differentiate from a common progenitor along 2 independent pathways that give rise to CD11c+ blood precursors with or without the expression of membrane CD1a[13, 14]. CD11c+CD1a+ DCs differentiate to Langerhans cells (LCs) in the epidermis and other epithelial surfaces, whereas the CD11c+CD1a− subtype replenishes various tissues with interstitial/tissue dendritic cells. The S100 positive and Cd1a negative cells observed in this patient are reminiscent of dendritic cells in RosaiDorfmandisease that are CD 68 and S-100 positive[15] We have shown that the patient described here had localized cutaneous Langerhans histiocytosis which was completely excised. A year later he developed nodular lesions in a different part of the skin. This proved to be a follicular lymphoma. The tumor was expressing H pylori antigen. Primary cutaneous lymphomas are usually regarded to represent the cutaneous counterpart of extranodal marginal zone lymphoma of the mucosa-associatedlymphoid tissue (MALT) (4-6).It has been described in several other tissues that include the lung, thyroid gland, conjunctiva, orbit and salivary glands[4-6]. The World Health Organization and the European Organization for Research and Treatment of Cancer (WHO-EORTC) have agreed on a new classification system[5]. The cutaneous B-cell lymphoma classifications designated by the WHO-EORTC include: primary cutaneous marginal zone B-cell lymphoma; primary cutaneous follicle center lymphoma; primary cutaneous diffuse large B-cell lymphoma, leg type; and primary cutaneous diffuse large B-cell lymphoma.Primary cutaneous marginal zone B-cell lymphoma (PCMZL), which includes the previously designated entities of immunocytoma, plasmacytoma, and extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoma tissue (MALT lymphoma), is a neoplasm of bcl-2+, CD10- and bcl-6- lymphocytes[4]. This immunophenotype facilitates differentiation of PCMZL from primary cutaneous follicular-center lymphomas and from cutaneous lymphoid hyperplasias.As far as we are aware this is the first cutaneous follicular B cell lymphoma associated with cutaneous H pylori infection. The tumor expressed H pylori antigen. It was incompletely excised but the residual tumor in the surgical wound regressed completely after anti H pylori drug treatment. The patient is alive and well a year later.
4. Conclusions
- Primary Cutaneous Follicular Lymphoma is type of skin cancer that has been dealt conventionally with radiotherapy or excision (surgically) and in some instances using chemotherapy. In this case, we use triple therapy (eradication of H. pylori). Using of triple therapy reveals that nonconventional relationship between H. pylori – host interactions that reflects future direction in etiology of pathogenesis as well as presenting potential strategies in treatment of these lymphomas- Use of Antibiotics in treatment of malignancy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML