Maytham M. Al-Hilo1, Shakir J. Al-Saedy1, Pers Y. Yacoob2
1MBChB, FICMS DV, Consultant Dermatologist and Venereologist, Al-Kindy Teaching Hospital, Baghdad, Iraq
2MBChB, Department of dermatology & Venereology, Al-Kindy Teaching Hospital, Baghdad, Iraq
Correspondence to: Maytham M. Al-Hilo, MBChB, FICMS DV, Consultant Dermatologist and Venereologist, Al-Kindy Teaching Hospital, Baghdad, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
This is a descriptive clinicopathological study (review of cases) that was carried out in the outpatient clinic of dermatology and venereology in Al-Kindy Teaching Hospital in Baghdad from December 2010 to April 2011. Forty-two patients with cicatricial alopecia were included in this study with age ranged from 17 to 62years with a mean age 35.07±11.23years.Primary cicatricial alopecia was seen in fourteen male patients (33.3%) and twenty-eight females (66.7%) making female to male ratio 2:1. Discoid lupus erythematosus was diagnosed in 17 patients (40.5%) with female to male ratio 4.6:1, their age ranged from 18-49 (32.2±8.44), lichen planopilaris was diagnosed in 12 patients (28.6%) with female to male ratio2:1, their age ranged from 17-62 years (41.613.29), pseudopelade of Brocq was diagnosed in 8 patients (19%) with female to male ratio 1.6:1, their age ranged from 22-53 years (33.7±9.53), folliculitis decalvans was diagnosed in 3 patients (7.1%), their age ranged from 21-31years (24.67±5.51), acne keloidalis nuchae was diagnosed in 2 male patients (4.8%), age ranged from 31-51(41±14.14).
Keywords:
Cicatricial Alopecia, Discoid Lupus, Folliculitis Decalvans, Pseudopelade, Keloidalis Nuchae, Lichen Planopilaris
Cite this paper: Maytham M. Al-Hilo, Shakir J. Al-Saedy, Pers Y. Yacoob, Primary Cicatricial Alopecia; A Clinical and Histopathological Descriptive Study, American Journal of Dermatology and Venereology, Vol. 2 No. 3, 2013, pp. 15-22. doi: 10.5923/j.ajdv.20130203.01.
1. Introduction
Primary cicatricial alopecia (PCA) isa group of disorders, in which hair follicle is the main target of destructive inflammation resulting in irreversible hair loss.[1]The exact pathogenesis of any of the PCAs is currently unknown. The destructive inflammatory changes in all primary cicatricial alopecia occur in the infundibular region and, to amore variable degree, the isthmic region of hair follicles.[2] All forms of PCA probably result from irreversible damage to the epithelial hair follicle stem cells that reside in a protected area of the outer root sheath called the bulge. The bulge is located in the upper third of the hair follicle at the level of the insertion of the insertion of the arrector pili muscle.[3]The histology is variable and non-specific.[4] The histological hallmark of a fully developed lesion is the replacement of the hair follicle structure by fibrous tissue.[3, 5&6]The most typical clinical manifestation of cicatricial alopecia is the loss of visible follicular ostia in a scarring area.[1, 3, 5&7] the lesions tend to be oval, and several foci may coalesce to form irregular bald areas. There is usually no erythema and the patches are smooth, shiny and slightly depressed.[4] Other signs of acute or chronic inflammation, including perifollicular erythema, perifollicular scale, crusting and pigmentary change may be present. Patient may report severe itching, pain and burning discomfort or may be entirely asymptomatic.[3]Clinical history, thorough examination of the entire scalp and skin biopsies can be crucial in the correct diagnosis of cicatricial alopecia. Diagnostic tools as a3-fold magnifying lens or a10-fold magnifying dermatoscope can help to identify the presence or absence of follicular ostia in the affected area.[8] the inflammatory nature of the lesions may only be evident on biopsy, a4-mm punch biopsy will provide the pathologist with an adequate specimen.[9] When indicated, adjunctive use of DIFparticularly in cases of primary lymphocytic cicatricial alopecia.[10] Special stains such as periodic acid-Schiff and mucin stains can further strengthen diagnostic conviction.[11, 12]At a recent workshop sponsored by the North American Hair Research Society (NAHRS), a working classification of primary cicatricial alopecia based on the predominant inflammatory cellular infiltrate was developed.[13] They classify PCA intolymphocytic which includes discoid lupus erythematosus, lichen planopilaris which further classified into (classic, Graham-Little syndrome and frontal fibrosing alopecia), pseudopelade of Brocq, central centrifugal cicatricial alopecia, alopecia mucinosa, keratosis pilaris spinulosa decalvans and morphoea/scleroderma.Neutrophilic which includes folliculitis decalvans and dissecting cellulitis. Mixed which includes acne keloidalis nuchae, acne necrotica and erosive pustular dermatosis and finally non specific or end stage cicatricial alopecia.
2. Materials and Method
This is a descriptive clinicohistopathological study (review of cases) carried out in the out-patient clinic of Dermatology and Venereology in Al-Kindy Teaching Hospital in Baghdad during the period from December 2010 to April 2011. This study was approved by the ethical committee of Al-Kindy Teaching Hospital. A written consent was taken from all of the patients before performing a biopsy. All patients with cicatricial alopecia were included in this study except those with secondary and congenital cicatricial alopecia. Forty two patients with cicatricial alopecia were included in this study, diagnosed clinically by two specialist dermatologist. The data were collected and analyzed by researcher using the Statistical Package for Social Science (SPSS, Version 17.0). Microsoft Office Excel was used for plotting graphs and table.
3. Results
3.1. Age and Gender Distribution
A total of 42 patients with primary cicatricial alopecia, their age varied between seventeen and sixty-two with a mean age (±SD) of 35.07 ± 11.23 years. Primary cicatricial alopecia was seen in fourteen male patients (33.3%). Their ages varied between 18 years to 62 years with mean age was 37.21±13.84 years. Female patients were twenty-eight (66.7%), their ages varied between 17 years and 61 years with a mean age was 34±9.78years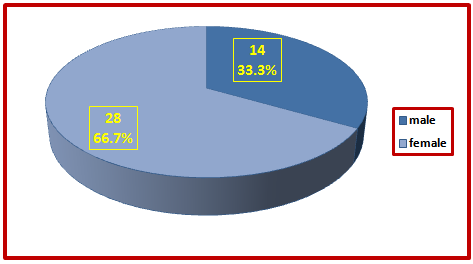 | Figure (1). Distribution of cases of PCA by gender |
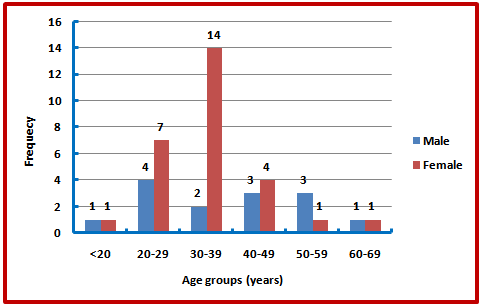 | Figure (2). Gender distribution of patients by age groups |
There was no statistically significant difference between the mean ages of both genders (P-value = 0.389). The age group most commonly affected was 30-39 years in 38.1% of patients. Chi-square test showed no statistically significant difference between age group distribution and sex of the patients (P-value = 0.215) (Figure 2). Least figure was in those with age group less than 20 years only one male patient and one female patient. Patients between (30-39) show the highest group in females (14) patients and 20-29 in males (4) patients.
3.2. Occupation of Patients
Their occupational history revealed that (71.4%) of male patients had engaged in outdoor occupation and (64.3%) of female patients were housewives.
3.3. Urban Versus Rural Distribution
It was observed that (76.2%) of patients came from Baghdad city and (23.8%) came from the rural areas related to it.
3.4. Duration of the Disease
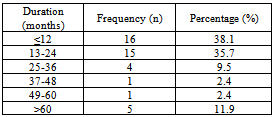
The mean duration of disease  was 2.3 ± 1.63 years, ranging from 2 months-10 years. The mean duration was 2.57±1.86 years in males and 2.17±1.51years in females. There was no statistically significant difference between the mean duration of disease in both genders (P-value = 0.468) (Table1).
was 2.3 ± 1.63 years, ranging from 2 months-10 years. The mean duration was 2.57±1.86 years in males and 2.17±1.51years in females. There was no statistically significant difference between the mean duration of disease in both genders (P-value = 0.468) (Table1).Table (1). Distribution of cases of PCA by duration of disease
 |
| |
|
3.5. Associated Symptoms
Increased shedding/hair loss, scalp itching, scale and pain were the 4 most common symptoms. All of the affected patients (100%) have hair loss, scalp itching (54.8%), scale (52.4%) and scalp pain (35.7%) (Figure 3):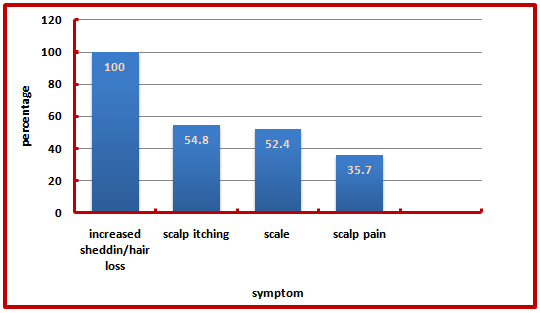 | Figure (3). Distribution of PCA by symptoms |
3.6. The Clinical Types
The most common type of PCA was DLE (40.5%) followed by other types shown in (figure 4):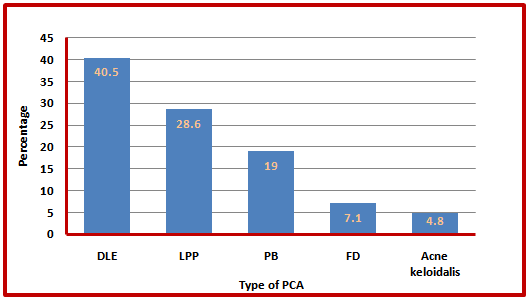 | Figure (4). Distribution of PCA by clinical types |
DLE was diagnosed in 17 patients. The female to male ratio was 4.6:1 (14 females and 3 males). The age at onset ranged from 18 to 49 years, with a mean age of 32.2 years ± 8.44. The average duration to diagnosis was 3.6 years (range: 4 months to 10 years with a median of 3 years).The 4 most common symptoms were increased shedding/hair loss (100%), scale (76.5%), itching (64.7%) and scalp pain (23.5%). Clinical presentationconsisted of erythematosus plaques of alopecia, atrophy, telangiectases, and follicular hyperkeratosis. The sites of involvement varied from patient to patient but frontal area of scalp was frequently involved (47.1%). The activity of the scalp disease was reflected by a positive hair pull test for anagen hairs and prominent follicular hyperkeratosis, particularly in the center of the plaque.A total of 5 patients (29.4%) with DLE had concomitant involvement of other body sites (figure 5).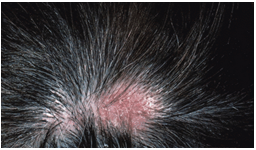 | Figure (5). discoid lupus erythematosus |
LPP was diagnosed 12 patients. The female to male ratio is 2:1(8 females and 4 males).The age at onset ranged from 17 to 62 with a mean age of 41.6 years ± 13.29. The average duration to diagnosis was 1.9(range: 2 months-8 years with a median of 6 months). The 4 most common symptoms were shedding/hair loss (100%), scale (75%), itching (91.7%) and scalp pain (58.3%). Clinical presentation consisted of solitary or multiple erythematosus plaques complicated by ulceration or atrophy. Activity predominantly in the periphery. The site of involvement varied from patient to patient but parietal area of the scalp was frequently involved (50%). Skin lesions of lichen planus was seen in (33.3%). In LPP one patient (8.3%) had oral lesions of lichen planus (white reticulate pattern). Nail changes (longitudinal ridging were observed in 2 patient (16.6%) (Figure 6).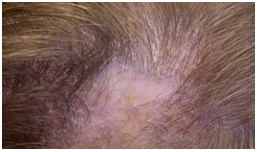 | Figure (6). lichen planopilaris |
Pseudopelade of Brocq was diagnosed in 8 patients. The female tomaleratio1.6:1 (5 females and 3 males).The age at onset ranged from 22 to 53, with mean age of 33.7±9.53.The average duration to diagnosis was 4 (range: 1-10 years with a median of 3years).The most common symptom was hair loss in 100%.Clinical presentation consisted of multiple small ovals to round or reticulated smooth, skin colored patches (figure7). 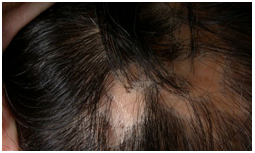 | Figure(7). Pseudopelade of Brocq |
Folliculitis decalvans was diagnosed in 3 patients. Age range 21-31 years with a mean age of 24.67±5.51, duration range 1-3 years. The most common symptom was pain, site of involvement was in vertex and occiput.Clinical presentation consisted of multiple follicular papules, pustules and plaques (figure 8).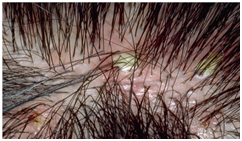 | Figure (8). Folliculitis decalvans |
Acne keloidalis nuchae was diagnosed in 2 males, age range 31-51 with a mean age of 41±14.14, duration range 1-2 years. The most common symptom was itching and pain, site of involvement was nape of the neck. Clinical presentation consisted of firm red-brown nodules and keloidal-like plaques (figure 9).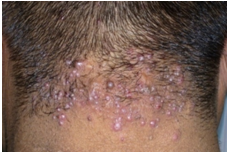 | Figure (9). Acne keloidalis nuchae |
3.7. Histopathological Characteristics of Primary Cicatricial Alopecia
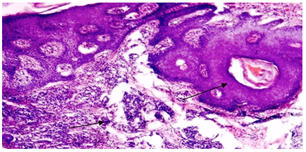
Repeated scalp biopsy specimens from new, active lesion may be necessary to make a definitive diagnosis. In all cases of DLE; histological examination was consistent with the diagnosis. Characteristic histological findingsincluded hyperkeratosis, epidermal atrophy, vacuolar degeneration of epidermal basal cells, follicular plugging, fibrous tract replacing follicle and a perivascular and periadnexial dermal lymphoid infiltrate (figure 10, 11). 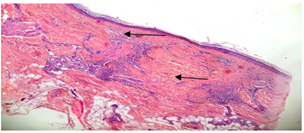 | Figure (10). hyperkeratosis,epidermalatrophy,fibrous tract replacement of hair follicle and perivascular lymphoid infiltrate (arrow heads) (haematoxylin- eosin; original magnification *10) |
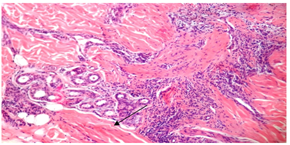 | Figure(11). periadnexial lymphoid infiltrate (arrow head) (H&E; original magnification *20) |
Characteristic histological findings in LLP included lichenoid interface dermatitis, follicular plugging, fibrous tract replacing follicle (cytoid bodies) and lymphocytic inflammatory infiltrate. (figure12, 13).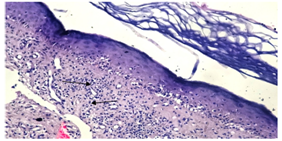 | Figure (12). lichenoid interface dermatitis and lymphocytic infiltrate (arrow heads)(H&E; original magnification*20) |
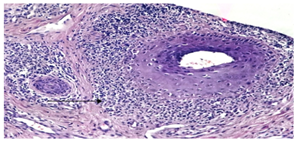 | Figure (13). perifollicular lymphoid infiltrate (arrow heads) (H&E; original magnification *20) |
Pseudopelade of Brocq fibrous tract replacing follicle and a lymphocytic infiltrate. (figure14).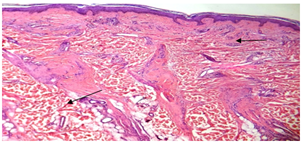 | Figure (14). fibrous tract replacing follicle and lymphocytic infiltrate(H&E; original magnification*10) |
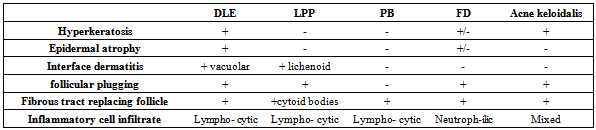
Folliculitis decalvans: hyperkeratosis or normal stratum corneum, normal or epidermal atrophy, follicular plugging, fibrous tract replacing follicle and neutrophilic infiltrate. (figure15).Table (2). Histopathological characteristics of primary cicatricial alopecias
 |
| |
|
 | Figure (15). follicular plugging and neutrophilic infiltrate(arrow heads)(H&E;original magnification*10) |
Acne keloidalis: hyperkeratosis, follicular plugging, fibrous tract replacing follicle and mixed cellular infiltrate. (figure16)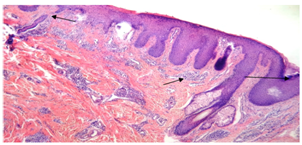 | Figure (16). follicular plugging, fibrous tract replacing follicle and mixed cellular infiltrate (arrow heads) (H&E; original magnification*10) |
Table (3). Distribution of cases of PCA by inflammatory cell infiltrate
 |
| |
|
4. Discussion
Primary cicatricial alopecia is a serious entity affecting on the self-esteem of the patient as the resultant alopecia is irreversible so early diagnosis and treatment is mandatory. The inflammatory process predominantly occurs around the permanent portion of the hair follicle (stem cells of the bulge area and the infundibulum), hence resulting in the irreversibility of alopecia.[14& 15] There is a paucity of data on cicatricial alopecia worldwide.[13& 14]In this study, PCA was more common in females than males (28 females and 14 males), making a female to male ratio of 2:1. This is in agreement with a study done in Canada (1.6:1)[16] and another study in Brazil (2.8:1)[17]and disagreed with the result of an Iranian study which reported a slightly higher male rate (1:108).[18] This can be attributed to social limitation or that the female patient does not visit the clinics as frequently as male.It was observed that (76.2%) of patients came from Baghdad city and (23.8%) came from the rural areas related to it. This difference may be related to higher levels of social, economical, cultural, hygienic and knowledge of people and easy access to medical centers in big cities.In our work the occupational history revealed that the majority (71.4%) of male patients had engaged in outdoor occupation. This may show the effect of environmental factors such as sun exposure in aggravating some types of PCA such as DLE and LPP.In the current study, the mean age of onset was 35.07 ±11.23years. The age group most commonly affected was 30-39 years in 38.1% of patients. These results are in agreement with those reported in the Iranian study in which the mean age of onset was 37.08 years[18], and in a Canadian study in which the mean age of onset `was 36.16 years.[19]The duration of PCA from onset to diagnosis ranged from 2 months to 10 years in our study. This result is close to the finding of an Italian study (3months to 8 years).[19]This study revealed that DLE was the most common type of PCA; it was present in 40.5% of cases, followed by LPP, Pseudopelade of Brocq, Folliculitis Decalvans and acne keloidalis nuchae respectively. These results are in agreement with the results of Iranian (25%), Canadian (33.9%), Brazilian (44.7%) and Indian (49%) studies in which DLE was the most common type[16-18&20]. However, LPP was the commonest type of PCA in Italian study constituting about 33.3% of cases followed by the DLE in 21.2% of cases.[19]In the current study, we reported a greater proportion of lymphocytic cicatricial alopecia versus neutrophilic/mixed cicatricial alopecia (7.4:1), which is in agreement with that reported in the Canadian (4:1), the Brazilian (6.6:1)and the Iranian study (1.86:1).[16-18]In accordance with previous studies;[14, 15&21]we observed a female predominance of lymphocytic cicatricial alopeciain which 27 patients (73%) were females and 10 patients (27%) were males, making a female to male ratio of 2.7:1. This is in agreement with Iranian (1.6:1), Brazilian (3.7:1) and Canadian (2.7:1) studies.[16-18]In our study, both LPP and DLE affected predominantly middle-aged adults, although LPP appears to have a later onset in life. In DLE, the activity of the disease is primarily focused in the center of the plaque, whereas in LPP the activity predominately occurred at the periphery.[14&16] Scalp pain and pruritus were more pronounced in LPP.A positive hair pull test assisted in determining the activity of disease.[14&22]The diagnostic challenge is in burnt-out stages of LPP and DLE when it is often clinically and histologically impossible to differentiate from other forms of cicatricial alopecia. [14&15] Repeated scalp biopsy specimens from new, active lesion may be necessary to make a definitive diagnosis. DLE of the scalp may precede, coexist, or follow other skin lesions.[23]In an earlier study by Wilson et al,[21] 34% of patients with DLE had scalp involvement and this may serve as a marker of chronicity and poor response to treatment. In a smaller study by Callen,[24] 41% of patients with DLE had cicatricial alopecia. In our series, approximately 29.4% of the patients with scalp DLE had lesions of DLE at other body sites apart from the scalp. This result is close to that observed in the study done in Canada (28.9%).[16]Classic presentation of lichen planus is not usually demonstrated on the scalp.[25-27]On the scalp; the classic clinical features include the presence of follicular hyperkeratotic papules, perifollicular erythema, and scaling at the periphery of the cicatricial process. Nail changes was recorded in (16.6%) of patients with LPP. This result is lower than that recorded in the Canadian study in which the nail changes were observed in (40%).[16]The term “pseudopelade” is confusing and denotes different meanings to different authors.[12,14,28&29] Sperling et al[27] has attempted to redefine pseudopelade of Brocq, follicular degeneration syndrome, and FD as subsets of central centrifugal scarring alopecia. In the recent NAHRS classification on cicatricial alopecia, classic pseudopelade of Brocq and central centrifugal alopecia belong to two distinct entities.[13] In our study, we defined Pseudopelade as both the classic reticulated footprints in snow pattern and the central variant. Pseudopelade may not be an uncommon occurrence in children and it is important to bear in mind this diagnosis whenever one encounters a child with patchy alopecia.[30] However, the majority of pseudopelade behaves in an insidious manner and is rarely inflammatory.[13&29]According to this work we noticed that pseudopelade is a great mimicker of many hair-loss conditions.[14&30] The central centrifugal form of pseudopelade can mimic AGA and we have seen at least 2 male patients inappropriately put on treatment for AGA by their referring physicians. The classic type of Brocq can mimic alopecia areata. In our series of patients, pseudopelade was misdiagnosed as alopecia areata, AGA, and other forms of scarring alopecia. Its noninflammatory nature frequently prevents early diagnosis. In accordance with previous studies,[15&30] This study revealed a male predominance of neutrophilic/mixed cicatricial alopecia with a male to female ratio of (4:1) which agreed with results seen in Iranian (4:1)[18] and Canadian (9.5:1)[16] and disagreed with the result seen in Brazilian study in which males and females were equally affected.[17]The pathogenesis of neutrophilic cicatrizing alopecia is unknown. Staphylococcus aureus is usually cultured from pustules but it is unclear whether this is a primary or secondary process. The possible role of bacterial superantigens or a defect in cell-mediated immunity has been postulated in neutrophilic cicatrizing alopecia.[14&15]Sites of occurrence may vary, but we observed preferential involvement of the posterior half of the scalp.Folliculitis keloidalispresents with follicular papules, plaques, and areas of fibrosis on the posterior scalp. From the histopathological point of view FD and folliculitis keloidalis may be two clinical types of the same spectrum. Underlying differences in follicular anatomy or host response may be responsible for the different clinical manifestations. Burnt-out or quiescent stages of neutrophilic cicatrizing alopecia have engendered some confusion with pseudopelade.
5. Conclusions and Recommendations
Cicatricial alopecia is an important entity and early diagnosis is mandatory, as the resultant alopecia is often irreversible.● An accurate diagnosis is achieved through a careful clinical and histopathologic evaluation.● PCA was more common in middle-aged adults and is more common in females.● DLE was the most common type of PCA.● There was a greater proportion of lymphocytic cicatricial alopecia versus neutrophilic/mixed cicatricial alopecia.● There was a female predominance of lymphocytic cicatricial alopecia and a male predominance of neutrophilic / mixed cicatricial alopecia.Amore detailed histopathological examination needs to be done using immunohistochemical and special stains.
References
| [1] | Harries MJ, Sinclair RD, Macdonald-Hull S, Whiting DA, Griffiths CE& Paus R. Management of primary cicatricial alopecias: options for treatment. Br J Dermatol. 2008; 159: 1-22. |
| [2] | Karnik P, Tekeste Z, McCormick TS, Gilliam AC, Price VH , Cooper KD& et al..Hair Follicle Stem Cell-Specific PPARy Deletion Causes Scarring Alopecia.J Invest Dermatol. 2009 May; 129(5): 1243-57. |
| [3] | Harries MJ, Trueb RM, Tosti A, Messenger AG, Chaudhry I, Whiting DA& et al. How not to get scar(r)ed: pointers to the correct diagnosis in patients with suspected primary cicatricial alopecia. Br J Dermatol. 2009; 160: 482-501. |
| [4] | Berker DAR, Messenger AG& Sinclair RD. Disorders of Hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. 8th ed. Blackwell Science. 2010. 66.2-66.39. |
| [5] | Ohyama M. Primary cicatricial alopecia. Recent advances in understanding and management. Journal of Dermatology. 2011; 38: 1-9. |
| [6] | Sperling LC. Scarring alopecia and the dermatopathologist. J CutanPathol. 2001; 28: 333-42. |
| [7] | Mirmirani P, Willey A, Headington JT, Stenn K, McCalmont TH& Price VH. Primary cicatricial alopecia: histopathologic findings do not distinguish clinical variants. J Am Acad Dermatol. 2005; 52: 637-43. |
| [8] | Otberg N, Wu WY, McElwee KJ& Shapiro J.Diagnosis and Management of Primary Cicatricial Alopecia: Part I. SKINmed:Dermatology for the Clinician.2008 January / February; 7(1): 19-26 |
| [9] | James WD, Berger TG& Elston DM. Andrews’ Diseases of the skin: clinical Dermatology. 10th ed. Canada, Elsevier: Saunder’s Company. 2006. 758. |
| [10] | Jordan R. Subtle clues to diagnosis by immunopathology: scarring alopecia. Am J Dermatopathol 1980;2:157-9. |
| [11] | Pinkus H. Differential patterns of elastic fibers in scarring and non-scarring alopecias. J Cutan Pathol 1978;5:93-104. |
| [12] | Elston DM, McCollough ML, Warschaw KE& Bergfeld WF. Elastic tissue in scars and alopecia. J Cutan Pathol 2000;27:147-52. |
| [13] | Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J , Sinclair R&et al. Summary of North American Hair Research Society (NAHRS)- sponsored workshop oncicatricial alopecia, Duke University Medical Center,February 10 &11, 2001. J Am Acad Dermatol. 2003; 48: 103-10. |
| [14] | Shapiro J. Cicatricial alopecias. In: Hair loss: principles of diagnosis and management of alopecia. London (UK): Martin Dunitz Ltd; 2002.155-74. |
| [15] | Whiting DA. Cicatrizing alopecia. Clin Dermatol. 2001; 19: 211-25. |
| [16] | Tan E, Martinka M, Ball N& Shapiro J. Primary cicatricial alopecias: Clinicopathology of 112 cases. J Am Acad Dermatol. 2004 Jan; 50 (1): 25-32. |
| [17] | Moure ERD, Romiti R, Machado MCMR& Valente NYS. Primary Cicatricial alopecias: a review of histopathologic findings in 38 Patients from a clinical university hospital in Sao Paulo, Brazil. Clinics. 2008; 63: 747-52. |
| [18] | Babaei Nejad Sh, Khodaeiani E& Amir Nia M. Descriptive study of primary Cicatricial Alopecia in patients referred to the Skin Clinic of Sina Hospital in Tabriz, 1997-2004. Iranian Journal of Dermatology. 2006 winter; 8(34): 496-500. |
| [19] | Amato L, Mei S, Massi D, Gallerani I& Fabbri P. Cicatricial alopecia;a dermatopathologic and immunopathologic study of 33 patients (pseudopelade of Brocq is not a specific clinic-pathologic entity).Int J Dermatol. 2002 Jan; 41(1): 8-15. |
| [20] | Inchara YK, Tirumalae R, Kavdia R& Antony M. Histopathology of scarring alopecia in Indian patients. Am J Dermatopathol. 2011 Jul; 33(5): 461-7. |
| [21] | Wilson CL, Burge SMS, Dean D&Dawber RPR. Scarring alopecia in discoid lupus erythematosus. Br J Dermatol. 1992; 126: 307-14. |
| [22] | Wiseman MC&Shapiro J. Scarring alopecia. J Cutan Med Surg. 1999; 3: 43-8. |
| [23] | Callen JP. Chronic cutaneous lupus erythematosus. Arch Dermatol. 1982;118: 412-16. |
| [24] | Callen JP. Systemic lupus erythematosus in patients with chronic cutaneous (discoid) lupus erythematosus. J Am Acad Dermatol. 1985; 12: 278-88. |
| [25] | Matta M, Kibbi AG, Khatter J, Salman SM& Zaynoun ST. Lichen planopilaris: a clinicopathologic study. J Am Acad Dermatol.1992;27:935-42. |
| [26] | Templeton SF& Solomon AR. Scarring alopecia. J Cutan Pathol.1993; 21:97-109. |
| [27] | Sperling LC, Solomon AR& Whiting RA. A new look at scarring alopecia. Arch Dermatol. 2000; 136: 235-42. |
| [28] | Dawber R. What is pseudopelade?. Clin Exp Dermatol. 1992; 17: 305-6. |
| [29] | Madani, Trotter MJ& Shapiro J. Pseudopelade of Brocq in the beard area. J Am Acad Dermatol. 2000; 42: 895-6. |
| [30] | Dinehart SM, Herzberg AJ, Kerns BJ& Pollack SV. Acne keloidalis. J Dermatol Surg Oncol. 1989; 15: 642-7. |



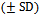 was 2.3 ± 1.63 years, ranging from 2 months-10 years. The mean duration was 2.57±1.86 years in males and 2.17±1.51years in females. There was no statistically significant difference between the mean duration of disease in both genders (P-value = 0.468) (Table1).
was 2.3 ± 1.63 years, ranging from 2 months-10 years. The mean duration was 2.57±1.86 years in males and 2.17±1.51years in females. There was no statistically significant difference between the mean duration of disease in both genders (P-value = 0.468) (Table1).













 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

