-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2013; 2(2): 10-14
doi:10.5923/j.ajdv.20130202.02
The Use of Konjac Glucomannan Hydrolysates (GMH) to Improve the Health of the Skin and Reduce Acne Vulgaris
Elaheh Bateni1, Richard Tester2, Farage Al-Ghazzewi2, Soheil Bateni3, Kamran Alvani2, John Piggott4
1Medical Office and Laser Clinic, Maryam Medical Complex, 20 Pars Ave., Shamsabadi Ave. Esfahan, Iran
2Glycologic Limited, 70 Cowcaddens Road, Glasgow G4 0BA, United Kingdom
3Samen Pharmacy, Amadegahave., Esfahan, Iran
4Strathclyde Institute of Pharmacy and Biomedical Sciences, Strathclyde University, Royal College, 204 George Street, Glasgow G1 1XW, United Kingdom
Correspondence to: Farage Al-Ghazzewi, Glycologic Limited, 70 Cowcaddens Road, Glasgow G4 0BA, United Kingdom.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Glucomannan hydrolysates (GMH) have been shown to have positive prebiotic properties and when ingested have been shown to have a positive but indirect impact on the skin. However, direct in vivo topical benefits have not been established. The aim of this work was to establish how effective GMH is with respect to improving skin health especially the reduction of infection due to acne vulgaris.Twenty six female volunteers aged between 18 to 39 years with active lesions of acne vulgaris were included in this study. Patients were assigned randomly into two groups to receive either a standard treatment or a spray formulation containing GMH at a concentration of 5% (w/v). Before and during treatment, the skin was evaluated according to the acne severity index (ASI). The results showed that there was a significant (P< 0.001) improvement of the skin health at the second (20 days) and third clinical evaluation (40 days) for established (e.g. antibiotics) and GMH treatments. Overall these data indicate that the GMH could be used as a prophylactic or novel topical therapeutic product for acne vulgaris and to improve skin health more generally.
Keywords: AcneVulgaris, Lesions, Skin Health, Konjac Glucomannan, Topical Therapeutic
Cite this paper: Elaheh Bateni, Richard Tester, Farage Al-Ghazzewi, Soheil Bateni, Kamran Alvani, John Piggott, The Use of Konjac Glucomannan Hydrolysates (GMH) to Improve the Health of the Skin and Reduce Acne Vulgaris, American Journal of Dermatology and Venereology, Vol. 2 No. 2, 2013, pp. 10-14. doi: 10.5923/j.ajdv.20130202.02.
Article Outline
1. Introduction
- Acne vulgaris, generally referred to as “acne”, is a multifactorial condition affecting skin areas containing the largest sebaceous gland follicles, including the face, back and trunk[1]. Even though the pathophysiology of acne is still not fully understood, it is believed to be related in part to excess sebum production, follicular hyperkeratinisation, hypercolonisation of the follicle with Propionibacterium acnes, inflammation and localized immune responses. Each of these factors may represent a potential therapeutic approach, and a variety of medications are available to use based on the type and severity of acne[2]. Treatments are based on topical (e.g. anti-infectious, topical retinoid or azelaic acid) and/or systemic treatments (e.g. antibiotics, hormonal therapy, zinc or isotretinoin)[3]. Various methods developed recently to assess the severity of acne vulgaris infection are focused on clinical examination[4]. Physicians usually describe the treatment of acne vulgaris in accordance to a classification scale of mild, moderate or severe[5]. The mild disease consists of open and closed comedones, moderate acne consists of more frequent papules and pustules with mild scarring and severe acne contains all of the above, in addition to nodular abscesses or cysts that leads to extensive scarring[6]. Mild acne requires generally topical treatment only[7]. However, moderate to severe acne requires systemic therapies such as antibiotics (e.g. tetracycline and minocycline), isotretinoin or hormone therapy for severe nodular acne[7, 8]. Effective acne management often requires the use of a combination of treatments that work on different parts of the pathogenic processes associated with acne development[9]. These therapies exhibit potential disadvantages and adverse effects on patients including gastrointestinal disturbances, vaginal candidiasis, hyperpigmentation[6] and the development of P. acnes and other bacterial resistance[10]. Hence, the need for alternative treatment strategies to reduce the use of antibiotics in acne therapy.Prebiotics in general and glucomannans in particular have been found to stimulate the immune system in vitro and in vivo[11, 12, 13, 14, 15]. Akiyama et al[16] reported that gluco-oligosaccharides can be used to control the growth of Staphylococcus aureus. More recently, it was found that konjac glucomannan hydrolysates (GMH) can stimulate the growth of probiotic microorganisms such as lactic acid bacteria (LAB) but inhibit the growth of undesirable ones such as Escherichia coli, Clostridium perfringens, Listeria monocytogens[11, 12], and Propionibacterium acnes[17]. Lactobacilli use different mechanisms to inhibit the growth of pathogens such as lowering pH (producing acids), excreting natural antibiotics, blocking pathogen adhesion and or competition for nutrients[18]. The authors recognise the significant physiological role that lactic acid bacteria play in the maintenance of the ecological balance of body cavities and skin ecosystem, particularly with respect to the availability of appropriate nutrients.Al-Ghazzewi and Tester[17] reported on the synbiotic ability of konjac glucomannan hydrolysates (GMH) to inhibit the growth of the skin bacterium Propionibacterium acnes in vitro. The authors concluded that all probiotic strains tested had the capability to inhibit the growth of this species of skin bacterium where the inhibition was significantly enhanced by the presence of the GMH prebiotic.The overall objectives of this (randomised) trial were to evaluate the efficacy of topical formulations (spray) containing konjac glucomannan hydrolysate as a novel future biotherapeutic agent in skin preparations for the treatment of acne vulgaris.
2. Materials and Method
2.1. Study Subjects
- A total of twenty six female volunteers aged between 18 to 39 years with active lesions of acne vulgaris were recruited for this clinical study. This investigation was conducted at the Esfahan Dermatology Clinic (Medical Office and Laser Clinic, Maryam Medical Complex, Esfahan, Iran). Facial skin condition was determined by a dermatologist on the basis of defining acne according to the type (comedonal, papulopustular or nodular) and severity (mild, moderate or severe) based on the number and type of lesions[19]. Participant consents were obtained before they were assigned randomly into two groups to receive either the standard treatment (e.g. topical or oral antibiotics, tretinoin, hormonal therapy, isotretinoin as well as cleaning soaps) or a formulation containing konjac glucomannan hydrolysate (Glycologic Limited, Glasgow, UK) at a concentration of 5% (w/v). Subjects were chosen on the following basis. Firstly, history of acne episodes for one year or more. Some of these patients had these conditions for 4 to 5 years. Secondly, a history of medical treatment such as those involving antibiotics prior to the study. Recruited patients used (i) the standard treatment or (ii) applied the GMH spray twice per day without other treatments except soaps for cleaning prior to application. Patients were examined by clinicians at assessments (appointments) every twenty days for a period of about six weeks (three appointments) for evaluation of the lesions and relevant side effects. Some patients came for more than three visits (data not included) – although the majority attended three times. Following treatment, the response was evaluated by the total count of acne lesions and by acne severity index (ASI). Acne severity was calculated[20] as follows:Acne severity index (ASI) = (1/4 x comedones) + (1 x papules) + (2 x pustules)
2.2. Statistical Analysis
- The data collected were analysed for statistical variance using Minitab 14 (Minitab Ltd., Coventry, UK). Differences are considered as statistically significant at P< 0.05.
3. Results
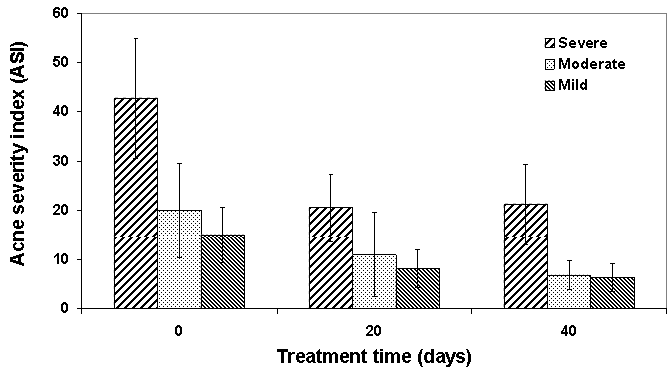 | Figure 1. Mean ± SD of effect of konjac glucomannan hydrolysates (GMH) on the treatment of patients with acne |
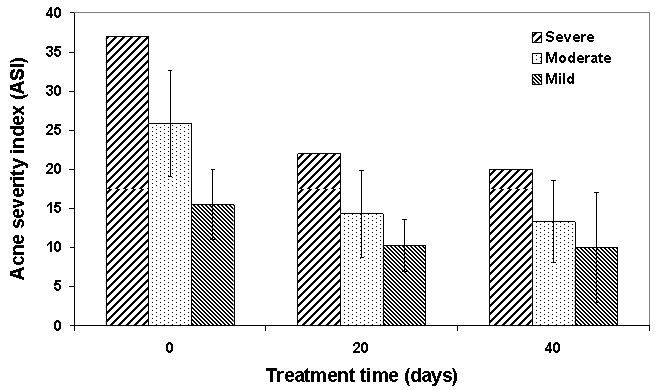 | Figure 2. Mean ± SD of effect of standard treatment on patients with acne |
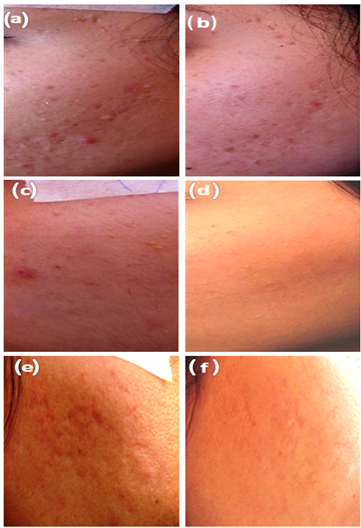 | Figure 3. Effect of konjac glucomannan hydrolysates (GMH) on facial acne in three patients before (a, c, e) and after (b, d, f) treatment for twenty days |
4. Discussion
- In this study, the acne severity index (ASI) was used to evaluate responses to acne treatment as this method gives better judgment about acne presence and changes during the treatment[20, 21]. Using this approach, every type of lesion has a different impact and is calculated for within the coefficient. The results showed a significant ability of GMH in improving acne severity index - as good if not better than other standard treatment medications available to patients. As acne has considerable psychological and emotional effects particularly among adolescence[22, 23], several medications are used for acne treatment. Depending on the severity, acne may require a combination therapy (two or more treatments) and this may include the use of a prescription topical `antimicrobial` or topical `retinoid`. For example, moderate to moderately severe acne usually requires the intervention of a dermatologist and combination therapy while severe acne is usually characterised by deep cysts, inflammation, extensive damage to the skin and scaring needing more extensive support. These infections can only be removed by physical methods and prescription medications used by dermatologists such as drainage and surgical excision, interlesional corticosteroid injection, isotretinoin, oral antibiotics or oral contraceptives. Many conventional types of medications, particularly antibiotics, have an adverse effect particularly in relation to the development of P. acnes and other bacterial resistance[10] and neuromuscular adverse effects associated with systemic retinoid dermatotherapy[24]. However, GMH is a natural product with no comparable adverse effect and can be used every day even as a prophylactic product. Glucomannans (gluco-oligosaccharides) have been reported to modulate skin bacterial proliferation and normalize sensitive skin barrier functionality[25]. A study by Suzuki et al[26] has concluded that consuming hydrolysed konjac glucomannan can help prevent atopic diseases by suppressing IgE production in mice and hence prevent the development of dermatitis. This study suggests that there is a possible link between the GMH and sebum gland physiology. It is possible that GMH may contribute toward unblocking the pores on skin surface and hence restore skin barrier properties and control superficial (and perhaps within sebaceous glands) microbial flora. Overall these data indicate that it would be worth examining further the inhibitory activities of the GMH and its interaction with natural skin flora with a view to employing it as an alternative therapeutic product for acne vulgaris.
5. Conclusions
- The use of topical formulations containing konjac glucomannan hydrolysate (GMH) was effective as a biotherapeutic agent for the treatment of mild to moderate acne in patients and as an adjuvant treatment for severe acne vulgaris.
ACKNOWLEDGEMENTS
- The authors would like to thank Dr E. Bateni for participating in this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML