-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2012; 1(3): 35-40
doi: 10.5923/j.ajdv.20120103.01
Epidemiological, Clinical and Cultural Study of Onychomycosis
K. Narotham Reddy1, B. Akshaya Srikanth2, T. Ram Sharan1, P. M. Biradar3
1Department of Dermatology, RIMS, Kadapa, India
2P. R. R. M.College of Pharmacy, Kadapa, India
3Department of Dermatology, M. R. M. C, Gulbarga, Karnataka, India
Correspondence to: K. Narotham Reddy, Department of Dermatology, RIMS, Kadapa, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
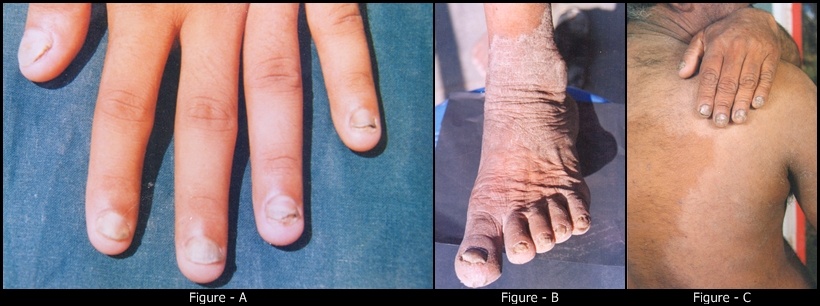
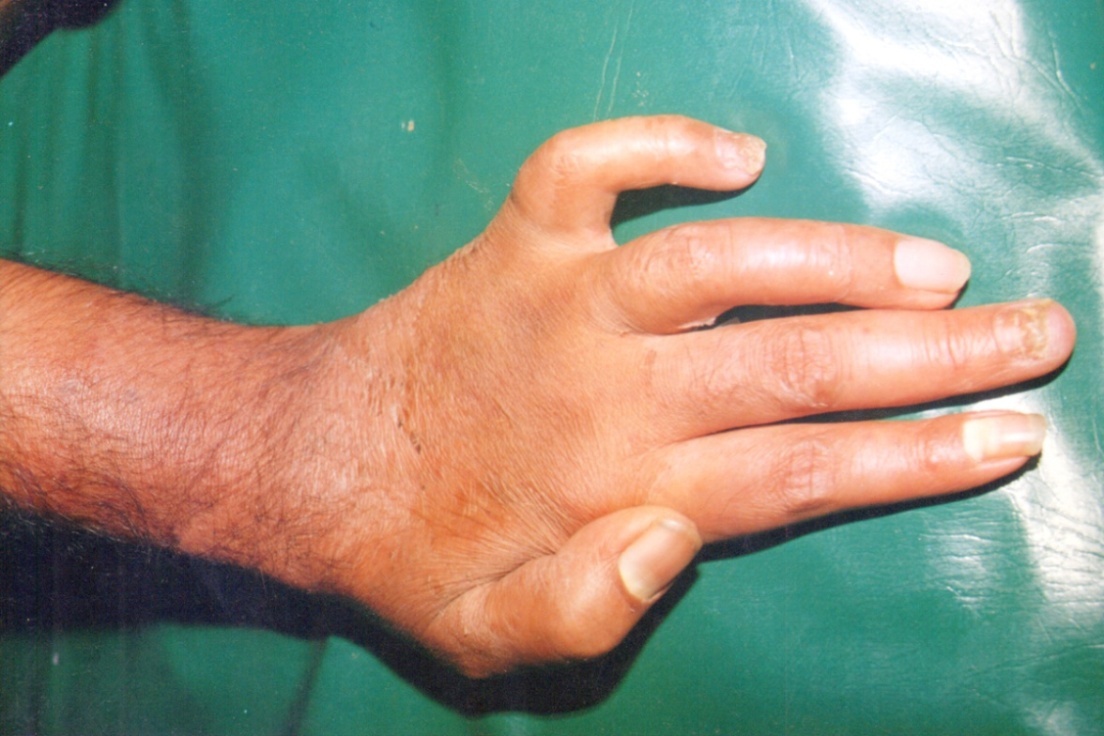
Background and Objective: Onychomycosis is a common nail disorder caused by dermatophytes and non-dermatophyte molds and represents most of the mycotic cutaneous infections. The purpose of the present study was to know the incidence of onychomycosis on both age and sex, morphological pattern of the disease, predisposing factors, associated conditions and identification of fungus by direct microscopy and by culture methods to rule out in Indian population. Methods: A prospective study was conducted on 64 patients of onychomycosis. A detailed history and thorough examination was done in all patients. The samples were taken from involved nails and skin and subjected to potassium hydroxide (KOH) examination and fungal culture and identification of fungal growth on Sabouraud’s dextrose agar (SDA) medium. Results: Distal sub-ungual onychomycosis (DSO) was commonest type 47(78.35%) patients, 10(16.6%) patients with candidial onychomycosis (CO), 2 (3.34%) patients with proximal subungual onychomycosis (PSO) and 1(1.71%) showing superficial white onychomycosis. The mycological observations showing positive fining with KOH were observed in 50(83%) and culture positive in 40(66.66%) cases. The most common predominant organism isolated is Trichophyton rubrum in 22(35%) cases followed by trichophyton mentagrophytes 6(10%) cases and the non-dermatophytes could be isolated in 3 cases (5%) only.
Keywords: Dermatophytes, Onychomycosis, Nails, Culture, Kadapa, India
Cite this paper: K. Narotham Reddy, B. Akshaya Srikanth, T. Ram Sharan, P. M. Biradar, "Epidemiological, Clinical and Cultural Study of Onychomycosis", American Journal of Dermatology and Venereology, Vol. 1 No. 3, 2012, pp. 35-40. doi: 10.5923/j.ajdv.20120103.01.
Article Outline
1. Introduction
- Fungal infections of the nails also called onychomycosis are commonest nail disorder including dermatophytes, non-dermatophyte moulds or yeasts[1]. It is one of the commonest nail disorders occurs in 30% of the superficial skin infections[2]. Tenia unguium is clinically defined as the dermatophytic infection of the nail plate[3]. Onychomycosis affects approximately 5% of the population worldwide[4] but variable frequency depending on different climatic, professional and socioeconomic conditions. It represents 20-40% of onychopathies and about 30% of mycotic cutaneous infections[5]. Various Indian workers have reported incidence to be at 0.5-5% in the general population[6]. Yeasts earlier regarded as contaminants are now increasingly being recognized as pathogens in the fingernails[7]. Onychomycosis is rarely life threatening, its high incidence and prevalence and the associated morbidity makes it an important public health problem. Dermatophytes cause 90% of toenails and 50% of the fingernail onychomycosis[8]. Candida species, particularly Candida albicans, prevail in finger infections[9]. Non-dermatophyte molds are rare, although few species are described as etiological agent of onychomycosis[2]. Onychomycosis can be clinically confirmed by direct microscopy of potassium hydroxide (KOH) preparation. However a fungal culture is required to identify the specific genus and species of pathogens[10]. The present study was planned to know the incidence of both age and sex, morphological pattern of the disease, predisposing factors, associated conditions and identification of fungus by direct microscopy and by culture methods to rule out the individual fungal species involved.
2. Materials and Methods
- This was a prospective study carried out from January 2011 to June 2012 in outpatients department and inpatients department of dermatology of RIMS hospital, Kadapa, A.P, India. The patients with clinical features of onychomycosis and microscopically proven to be positive for fungal elements were taken up for the study. A detailed history of every patient along with complaints and duration of disease was taken and noted in a specially designed case report proforma. History regards demographic details, family history, nature of onset, duration of treatment taken was collected. All the nails were examined in good light precise involvement of the nails and morphology of the disease. A detailed general physical, mucocutaneous examination was conducted in all patients. The physical examination features of the affected nails and nail folds were noted and recorded in a table form. All the patients were examined for any superficial fungal infections of the skin and any other associated skin or systemic disease especially psoriasis, lichen planus, HIV, and diabetes mellitus.The suspected nails were cleaned with 70% alcohol and nails scrapings were taken with a sterile scalpel blade and collected in a black sterile paper using a sterile nail cutter or scarper. The samples were also taken also taken from the associated fungal infections of skin and nail folds in cases of paronychia. The screening of samples was done by direct microscopy with 10% KOH were slightly warmed whenever required for rapid dissolution of the materials. The softened nail materials were examined under both low and high power of the microscope for the presence of fungal elements. The details regarding the hyphae, spores, budding cells and pseudo-hyphae were noted.The samples were incubated by using sterile spud 10-20 pieces of the materials are inoculated on the surface of Sabouraud’s Dextrose Agar (SDA) and inoculated at 37℃ for 2-4 days for isolation of yeasts, 26°C for 14-30 days for moulds/dermatophytes. SDA with chloramphenicol (5gms) + Cycloheximide (50gms) is used for isolation of dermatophytes. The morphological characteristic of the rate of the growth, colony size, shape, margins, colour of the colony, type of growth and the pigment produced on reverse were carefully observed and noted. Nail samples from 8 cases of onychomycosis have been selected for histopathological examination by hematoxylin and Eosin staining (H&E) and periodic acid-Schiff staining (PAS).
3. Results
|
|
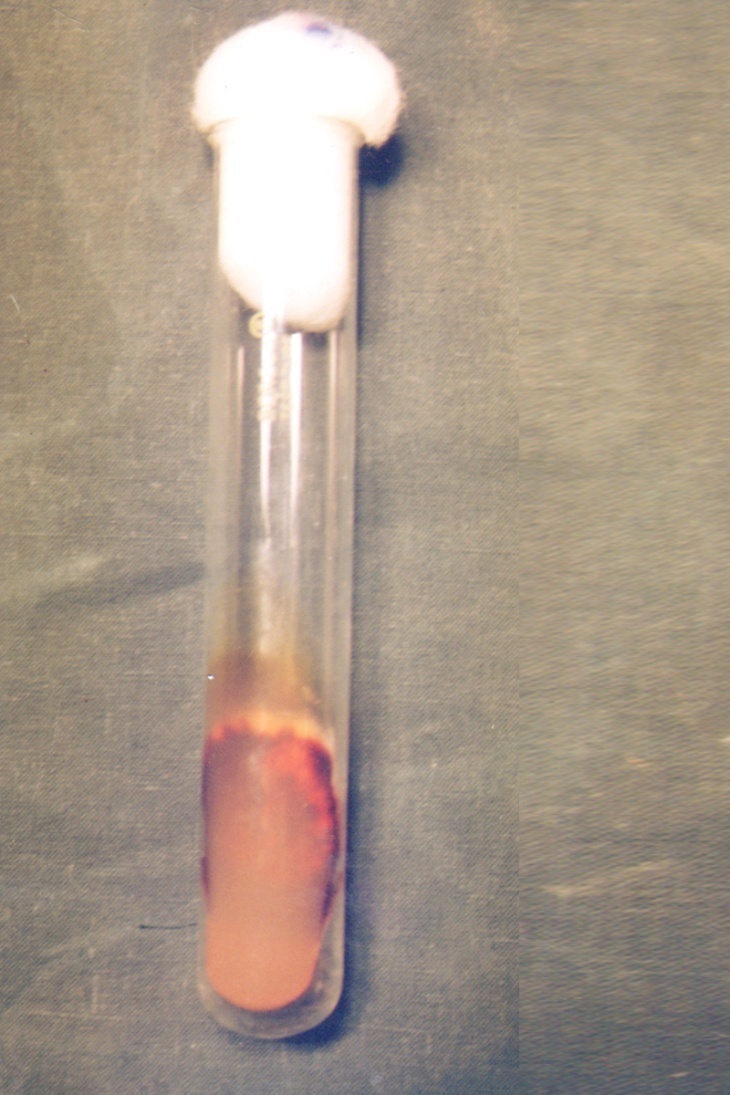
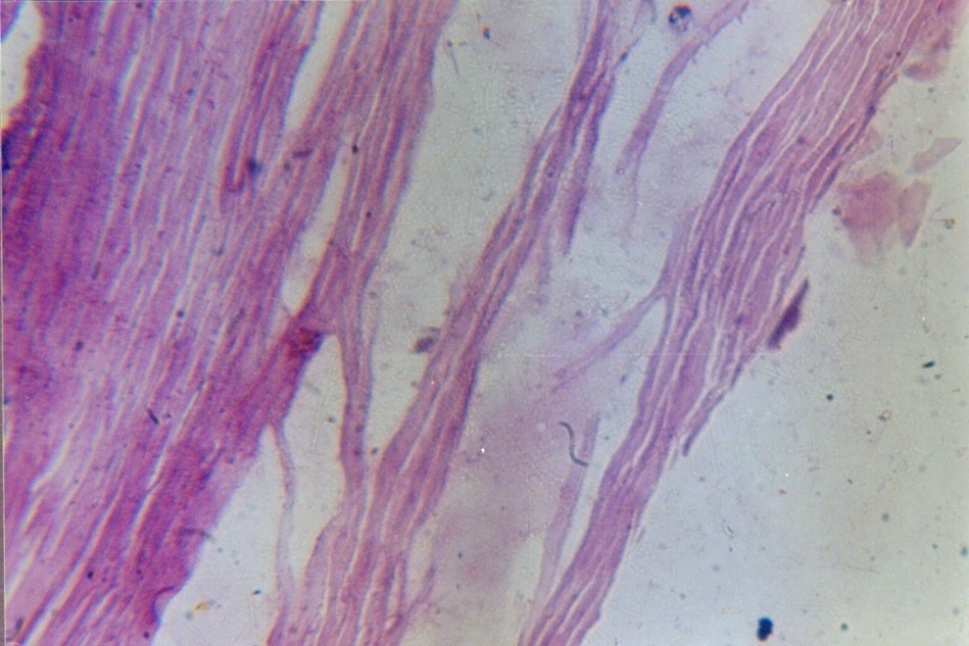 | Figure 3. Trichophyton rubrum – Sabouraud dextrose agar (with double antibiotic) showing reverse view of colony of Trichophyton rubrum showing red wine pigmentation |
 | Figure 4. H&E – 280X: H & E stain of Fungal Nail |
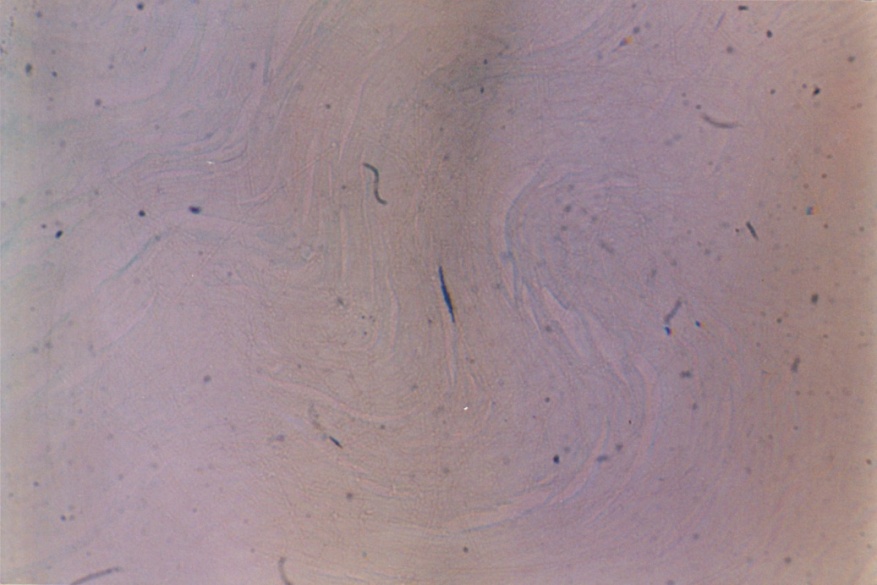 | Figure 5. Grocott’s stain – 280X: Black fungal hyphae are observed |
4. Discussion
- Onychomycosis affects all age groups. In our study 36.70% of patients were between 21-30 years, 1% below 20 years and 3.33% above 60 years of age. Te overall prevalence of onychomycosis in children has been reported 0.44%[11]. The low prevalence of onychomycosis in children is attributed to difference in nail plate structure, lack of cumulative trauma and increased growth rate of nail plate with subsequent elimination of fungus[12]. There are mixed reports about the prevalence of onychomycosis in adults and elders. Some authors reported a high prevalence in the age group of 20-40 years[13,14,15] while others have reported a high prevalence above 55 years of age[16,17]. The incidence of onychomycosis may be higher in elderly in India but the disease being mostly asymptomatic and elderly being dependent upon for medical help may not be presenting to the hospital. Onychomycosis in our study was found to be more common in males 65% than females 35%. This has been found observed by most of the workers from India and abroad[13,18,19]. However distal subungual onychomycosis was more common in females. This has been attributed to greater burden of wet work with increased trauma facilitating easy entry to fungal pathogens[10,20]. In our study the common predisposing factor preceding to the development of onychomycosis observed were associated with wet work 43.3%. The predisposing factor of our study correlates with the study reported by Madhuri et al[21]. In this study common clinical presentation observed in all types of onychomycosis was nail discoloration (100%) and subungual hyperkeratosis in 93%. This concurs with the study done by Ramesh et al[22]. The housewives formed of the largest group (26.6%) in our study and this could be associated with wet work associated with constant trauma could explain the probable reason for the high prevalence of onychomycosis in the wet work occupation workers (43.3%). Increased physical activity (26.6%) with trauma facilitating easy entry of the fungal pathogens could probably explain the second contributing factors. In present study only finger nails were involved in 33(55%) patients, only toe nails in 24(40%) and both finger and toe nails in 3 (5%). Most of the house wives from India show that fingernail onychomycosis is more common than toe nail onychomycosis[14,23]. Sabouraud’s Dextrose Agar (SDA) culture medium was used for the isolation of fungi and the growth rate on SDA were 83% for dermatophyte growth observed in first two weeks of inoculation. The most common isolate obtained in our study was Trichophyton rubram. It has been reported as most prevalent pathogen in onychomycosis by many workers[24,25]. The high rate of isolation of T.rubrum can be explained on the basis that can be explained on the basis that it has greater capacity to infect the nails because it can easily colonise on the hard keratin[26,27].Trichophyton mentagrophyte is the second most common isolated dermatophyte (6.66%) in our study. It was isolated from both finger and toe nails onychomycosis. Although T. mentagrophyte has been reported to be usually associated with toe nail onychomycosis[23] but T.rubrum is still more common than T.mentagrophyte in the toe nail onychomycosis. Out of four cases of distal subungual onychomycosis (DSO), the isolate obtained from 47 cases were shown positive towards T. rubrum and T.mentagrophytes. This is the commonest dermatophyte implemented in the etiology of distal subungual onychomycosis (DSO)[9]. There were 4 cases (6.66%) of Candida albicans in our study. All these patients had chronic paronychia with nail and toe involvement. Among all the yeast, Candida species are reported most frequently reported[14,28].The non-dermatophytes could be isolated in 3 cases (5%) only. The only non-dermatophytes isolate was Aspergillus flavum from 2 cases of finger nail and 1 case of Aspergillus niger of finger nail. Aspergillus sp. has earlier been reported from India as the cause of onychomycosis[29]. In our study there were 9 (15%) cases of mixed infections of T. rubrum and Aspergillus flavum. Mixed infections are described in the literature but are uncommon[21]. The most possible explanation for mixed etiology is that the disease and dystrophic nails already damaged by dermatophytes than non-dermatophytes.
5. Conclusions
- Onychomycosis is caused by the dermatophytes of fungi and non-dermatophytes moulds. Trichophyton rubrum was most common isolate. Although the onychomycosis is not a life threatening, it can be source of pain and discomfort; it can also pose a risk for the patients, their families and others in contact with them. Onychomycosis can no longer consider a simple cosmetic nuisance confined to the nails. It is a significant and important disease, which can generate many physical, psychological and occupational problems considerably impairing patient’s quality of life.
ACKNOWLEDGEMENTS
- The authors thank dermatology staff of RIMS Medical College, Kadapa, for their continuous support and encouragement.
References
| [1] | Weitzman I, Surnmerbell RC. The dermatophytes. Clin Microbiol Rev 1995;8:240-59 |
| [2] | Andre J, Achten G. Onychomycosis. Int J Dermatol 1987; 26: 481-490. |
| [3] | IADVL Textbook and atlas of dermatology , Chetan Oberoi, AK Miskeen, Superficial fungal infections 1994;1:184-185. |
| [4] | Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol 2002;43”105-12. |
| [5] | Achten G, Wanet RJ. Onychomycosis in the laboratory Mykosen 1978;21:125-7. |
| [6] | Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: Guntur and their response to treatment with Hamycin, Dermostatin and Dermamycin. Indian J Dermatol Venereol Leprol. 1970;36:209–14. |
| [7] | Karmakar S, Kalla G, Joshi KR, Karmakar S. Dermatophytosis in a desert district of Western Rajasthan. Indian J Dermatol Venereal Leprol. 1995;61:280–3 |
| [8] | Elewski BE. Onychomycosis: pathogenesis, diagnosis and management. Clin Microbiol Rev.1998;11:415–29. |
| [9] | Kaur R, Kashyap B, Bhalla P. Onychomycosis-epidemiology diagnosis and management. Indian J Med Microbiol 2008;26:108-16. |
| [10] | Chander J. Muscellaneous opportunistic mycosis. In: Chander J. editor. Textbook of Medical Mycology. 3rd ed. New Delhi;Mehta publishers;2009.p.388-90. |
| [11] | Ahmed M, Gupta S, Gupta S. A Clinico-mycological study of onychomycosis Egyptian Dermtol Online J 2010;6(1):4. |
| [12] | Tisti A, Piraccini BM, Lorenzi S. Onychomycosis caused by non-dermatophytes: Clinical features and response to treatment of 59 cases. J Am Acad Dermatol 200;42:217-24. |
| [13] | Jain S, Sehgal VN. Onychomycosis: an epidemio-etiologic perspective. Int J Dermatol 2000;39:100-113. |
| [14] | Philpot CM, Shuttleworth D. Dermatophyte onychomycosis in children. Clin Exp Dermatol 1989;14:203. |
| [15] | Ramesh V, Singh R, Reddy BSN, Kumari S. Clinico-mycological study of onychomycosis. Indian J Dermatology Venerol Lepreol 1982;48(3):145-150. |
| [16] | Grover S. Clinicomycological evaluation of onychomycosis at Bangalore and Jorhat. Indian J Dermatol Venereol Leprol 2003;69:284-286. |
| [17] | Banerjee U, Sethi M, Pasricha JS. Study of onychomycosis in India. Mycosi 1989;33(7/8):411-415. |
| [18] | Raberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol 1992;126(Suppl.39):23-27. |
| [19] | Hay RJ. Onychomycosis. Dermatol Clin 1993; 11(1):167. |
| [20] | Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dematol 1994; 130(Suppl. 43):15. |
| [21] | Reddy BSN, Singh G, Sharma BM. Onychomycosis and nail dystrophy. Indian J Dermatol 1977;23(1):2. |
| [22] | Madhuri T, Jesudanam TM, Rama Rao GR, Lakshmi DJ. Onychomycosis: a significant medical problem. Indian J Dermatol Venereol Leprol 2002; 68:326-329. |
| [23] | Ramesh V, Singh Rattan, Reddy BSN and Kumari Sud. “Clinicomycological study of onychomycosis”. Indian J Dermatol Venereol Leprol 1982; 48:145-150. |
| [24] | Puri DKK, Sarin RC, Arora S. Pattern of dermatophytes affecting the nails. Indian J Dermatol Venereol Leprol 1978;44(2):91-94. |
| [25] | Vinod S, Grover S, Dash K. A clinic-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol 2000;66:238-240. |
| [26] | Clayton YM. Clinical and mycological aspects of onychomycosis and dermatomycosis. Clin Exp Dermatol 1992;14(Suppl):37. |
| [27] | Hay RJ. Onychomycosis. Dermatol Clin 1993;11(1):167. |
| [28] | English MP. Nails and fungi. Br J Dermatol 1976;94:697. |
| [29] | Haneke E, Achten G. Onychomycosis. Int J Dermatol 1987;26:481-490. |
| [30] | Ramani R, Srinivas CR, Ramani A, Kumari TG, Shiv ananda PG. Moulds in onychomycosis. Int J Dermatol 1993;32:877-878. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML